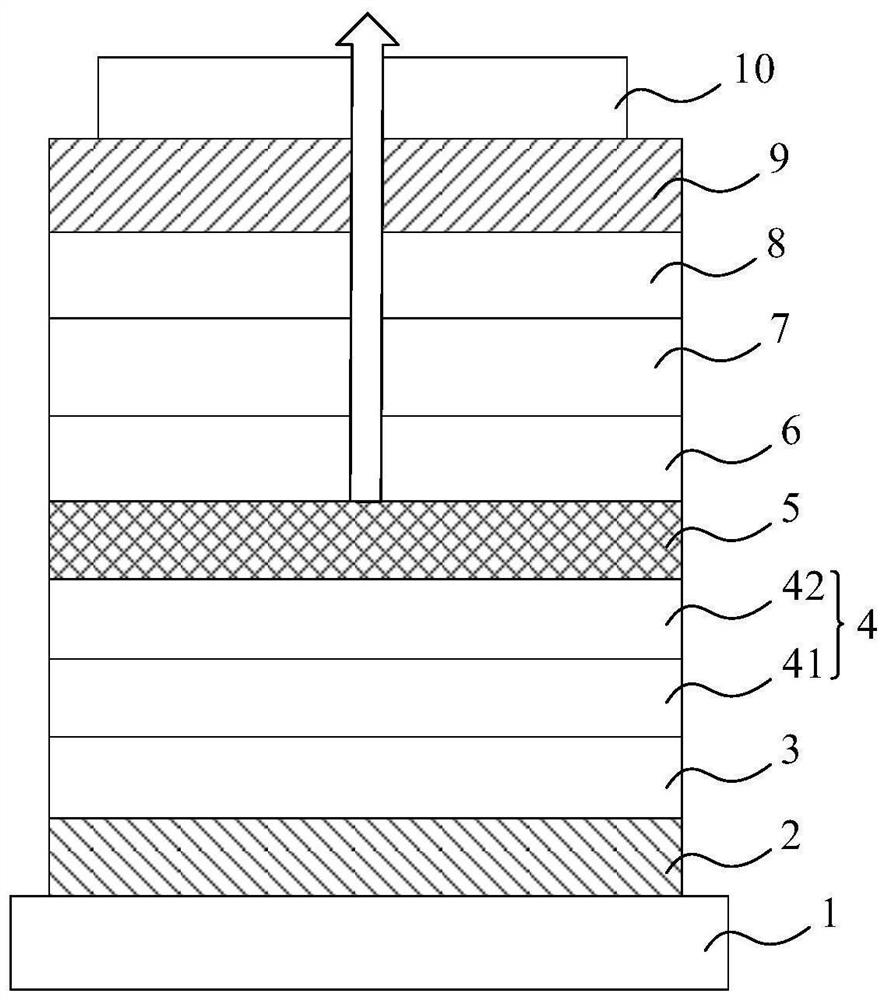
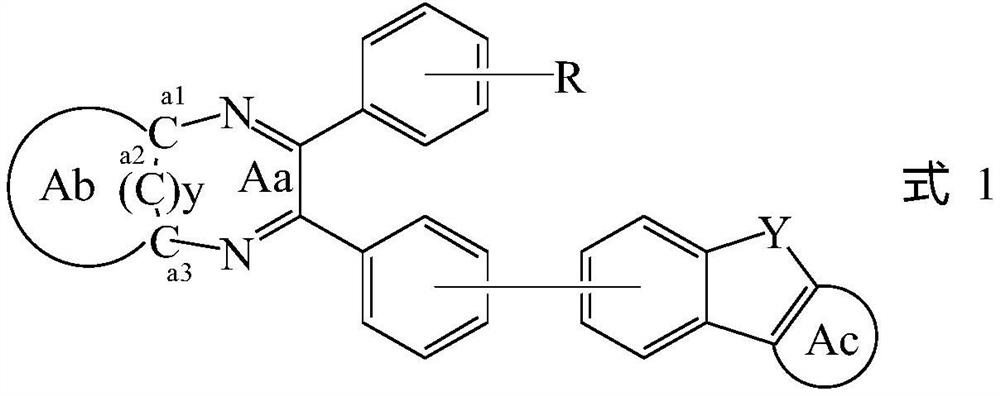
Compound, display panel and display device
A compound and connection position technology, applied in organic chemistry, chemical instruments and methods, luminescent materials, etc., can solve the problems affecting OLED luminous efficiency, lower exciton formation efficiency, low electron transport ability, etc., to achieve inhibition of electron transport performance and Reduced exciton formation efficiency, enhanced electron injection and transport capabilities, and effects of high exciton utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0176] Example 1: Preparation of compound J53
[0177] (One)
[0178] Under a nitrogen atmosphere, o-phenylenediamine (8.8mmol) and 4,4'-dibromobiphenyl (8mmol) were added to 80mL of acetic acid, stirred and heated, and reacted at 100°C for 24h. After the reaction is over, cool to room temperature, pour it into ice water with stirring, perform suction filtration, collect the filter cake, dissolve it in dichloromethane, and add water for extraction, collect the organic phase and use anhydrous Na 2 SO 4 Dry, collect the filtrate by suction filtration, spin off the solvent and perform column chromatography purification to obtain the intermediate Me-1 (yield 85%).
[0179] LC-MS: m / z: calculated value: C20H12Br2N2: 440.13, measured value: 439.89.
[0180] (two)
[0181] Under nitrogen atmosphere, add 100 mL of toluene: ethanol: water (10:1:1) mixed solvent into a 250 mL reaction flask, and then add K in sequence 2 CO 3 (2.5mmol), intermediate Me-1 (1mmol), dibenzofuran-4-boronic acid (1m...
Embodiment 2
[0190] Example 2: Preparation of compound J34
[0191] (One)
[0192] The preparation method of intermediate Me-4 and Me-3 is similar, the difference is that the Re-1 in step (3) of Example 1 is replaced with an equimolar amount of Re-2, and finally intermediate Me-4 is obtained (yield 65%).
[0193] LC-MS: m / z: calculated value: C29H20BN3O2: 453.30, measured value: 453.09.
[0194] (two)
[0195] The preparation method of compound J34 is similar to that of J53, except that Me-3 in step (4) of Example 1 is replaced with an equimolar amount of Me-4, and finally compound J34 is obtained (yield 80%).
[0196] LC-MS: m / z: calculated value: C61H37N5O: 855.98, measured value: 855.67.
[0197] Elemental analysis results of the compound: calculated value: C61H37N5O (%): C 85.59, H 4.36, N 8.18; test value: C 85.60, H 4.35, N 8.20.
Embodiment 3
[0198] Example 3: Preparation of Compound J12
[0199] (One)
[0200] The preparation method of intermediate Me-5 and Me-3 is similar, the difference is that the Re-1 in step (3) of Example 1 is replaced with an equimolar amount of Re-3, and finally intermediate Me-5 is obtained (yield 62%).
[0201] LC-MS: m / z: calculated value: C29H20BN3O2: 453.30, measured value: 453.12.
[0202] (two)
[0203] The preparation method of compound J33 is similar to that of J53, except that Me-3 in step (4) of Example 1 is replaced with an equimolar amount of Me-5 to finally obtain compound J12 (yield 77%).
[0204] LC-MS: m / z: calculated value: C61H37N5O: 855.98, measured value: 855.63.
[0205] Elemental analysis results of the compound: calculated value: C61H37N5O (%): C 85.59, H 4.36, N 8.18; test value: C 85.58, H 4.35, N 8.21.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com