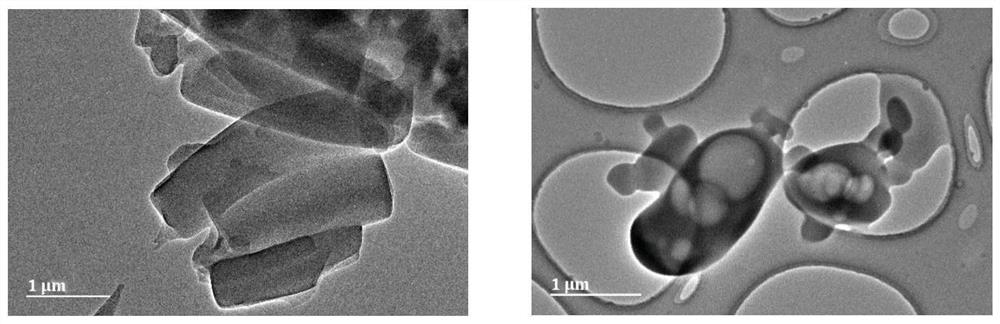
Calcium granule, and preparation method and application thereof
A technology of granules and calcium salts, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve the problems of inconvenient to carry, fragile, difficult to dissolve calcium gluconate particles, etc. , to achieve the effect of slow dissolution rate, short dissolution time, good stability and taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of embodiment 1 compound calcium gluconate granules
[0068] 1.1 Preparation of samples 1-5
[0069] 1) Take sucrose, grind it to -80 mesh, and set aside;
[0070] 2) Take 1.2L of purified water, heat to boil, add 0.5kg of calcium gluconate, 0.5kg of calcium lactate and 0.05kg of lactic acid in sequence, continue heating, stir to dissolve, add different amounts of water, stir and mix well, and obtain a solid content of 30% to 60% of granulation liquid;
[0071] 3) Add 2 kg of pulverized sucrose to the fluidized bed as solid powder for granulation, start the fluidized bed, see Table 1 for the granulation parameters, and spray the granulation solution obtained in [step 2) into the binder solution respectively, Granulate and discharge to obtain compound calcium gluconate granule samples 1-5. See Table 2 for the solid content of the granulation solution used in each sample.
[0072] Table 1 Granulation parameters
[0073]
[0074] 1.2 Preparation of s...
Embodiment 2
[0090] The preparation 2 of embodiment 2 compound calcium gluconate granules
[0091] 1) Take sucrose, grind it to -80 mesh, and set aside;
[0092] 2) Take 1.2L of purified water, heat to boil, add 0.5kg of calcium gluconate, 0.5kg of calcium lactate and 0.05kg of lactic acid in sequence, continue heating, stir to dissolve, add purified water, control the solid content to 47%, stir and mix, to obtain granulation liquid;
[0093] 3) Add 2 kg of pulverized sucrose powder in the fluidized bed as granulated solid powder, open the fluidized bed, set the intake air volume, injection air pressure and spraying rate of the injection stage according to the data listed in Table 5 , and other granulation parameters are shown in Table 4, respectively sprayed into the granulation solution obtained in the binder solution [step 2), granulated, and discharged to obtain compound calcium gluconate granule samples 7-11.
[0094] Table 4 Granulation parameters
[0095]
[0096] The granulat...
Embodiment 3
[0100] The preparation of embodiment 3 compound calcium gluconate granules 3
[0101] 1) Take sucrose, grind it to -80 mesh, and set aside;
[0102] 2) Take an appropriate amount of purified water, heat to boil, add calcium gluconate, calcium lactate and lactic acid in the prescription amount listed in Table 6, continue heating, stir to dissolve, add purified water, control the solid content to 47%, stir and mix, to obtain granulation liquid;
[0103]3) Add 2 kg of pulverized sucrose powder into the fluidized bed as solid powder for granulation, start the fluidized bed, see Table 1 for the granulation parameters, and spray the granulation solution obtained in [step 2) into the binder solution respectively, Granulate and discharge to obtain compound calcium gluconate granule samples 12-19.
[0104] Take the above 8 samples respectively, measure the dissolution time, observe the properties, and taste the mouthfeel. The results are shown in Table 6.
[0105] Table 6
[0106] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com