Lithium-rich manganese-based positive electrode material capable of realizing accurate lithium matching, and preparation method and application thereof
A positive electrode material, lithium-rich manganese-based technology, applied in the field of lithium-rich manganese-based positive electrode materials and its preparation, can solve the problems of single composition structure, large error in lithium allocation, etc., achieve stable electrochemical performance, prevent particle breakage, Conducive to the effect of circulatory stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A lithium-rich manganese-based positive electrode material for realizing precise lithium allocation, the preparation method of which comprises the following steps:
[0047] S1. Weigh manganese sulfate and nickel sulfate with a molar ratio of 3:1 and mix them, add distilled water to make a 2mol / L metal salt solution (the total molar number of transition metals is 2mol), then weigh 4mol sodium hydroxide to make 4mol / L solution, using ammonia water as a complexing agent to control the release of metal ions in the solution;
[0048] S2. Slowly add the metal salt solution, the precipitating agent solution and the complexing agent into the reaction kettle, control the reaction temperature at 50-60°C, react for 10-12 hours, filter to obtain a precipitate, and vacuum-dry to obtain a hydroxide precursor;
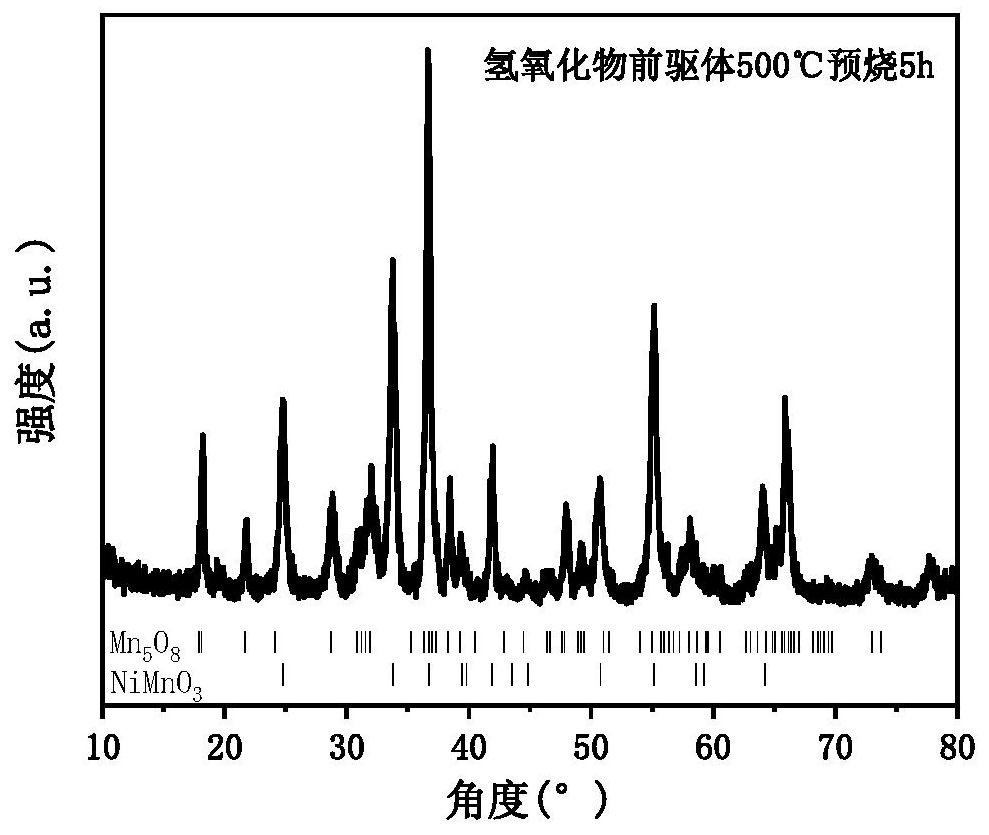
[0049] S3, putting the hydroxide precursor into a muffle furnace, and calcining at 500° C. for 5 hours to obtain an oxide precursor;
[0050] S4. Mix and stir the oxide precu...
Embodiment 2
[0057] A lithium-rich manganese-based positive electrode material for realizing precise lithium allocation, the preparation method of which comprises the following steps:
[0058] S1. Weigh manganese sulfate and nickel sulfate with a molar ratio of 3:1 and mix them, add distilled water to make a 2mol / L metal salt solution (the total molar number of transition metals is 2mol), then weigh 4mol sodium hydroxide to make 4mol / L solution, using ammonia water as a complexing agent to control the release of metal ions in the solution;
[0059] S2. Slowly add the metal salt solution, the precipitating agent solution and the complexing agent into the reaction kettle, control the reaction temperature at 50-60°C, react for 10-12 hours, filter to obtain a precipitate, and vacuum-dry to obtain a hydroxide precursor;
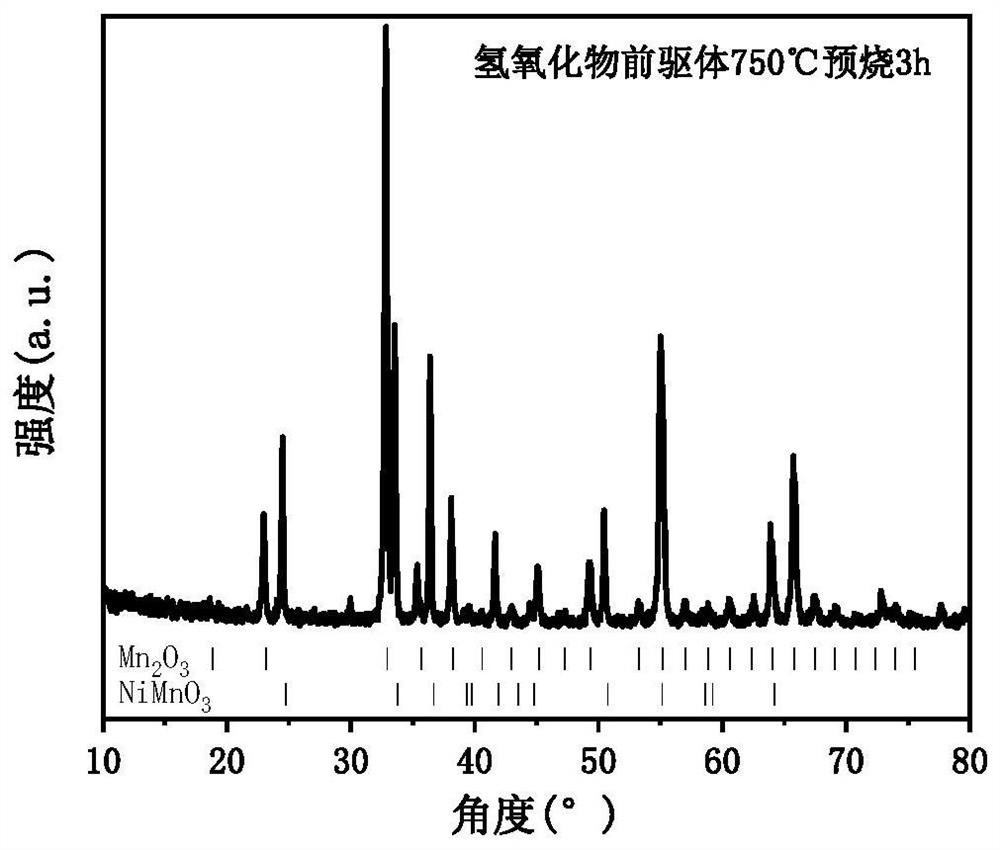
[0060] S3, putting the hydroxide precursor into a muffle furnace, and calcining at 750° C. for 3 hours to obtain an oxide precursor;
[0061] S4. Mix and stir the oxide precu...
Embodiment 3
[0067] A lithium-rich manganese-based positive electrode material for realizing precise lithium allocation, the preparation method of which comprises the following steps:
[0068] S1. Weigh manganese sulfate and nickel sulfate with a molar ratio of 3:1 and mix them, add distilled water to make a 2mol / L metal salt solution (the total molar number of transition metals is 2mol), then weigh 4mol sodium hydroxide to make 4mol / L solution, using ammonia water as a complexing agent to control the release of metal ions in the solution;
[0069] S2. Slowly add the metal salt solution, the precipitating agent solution and the complexing agent into the reaction kettle, control the reaction temperature at 50-60°C, react for 10-12 hours, filter to obtain a precipitate, and vacuum-dry to obtain a hydroxide precursor;
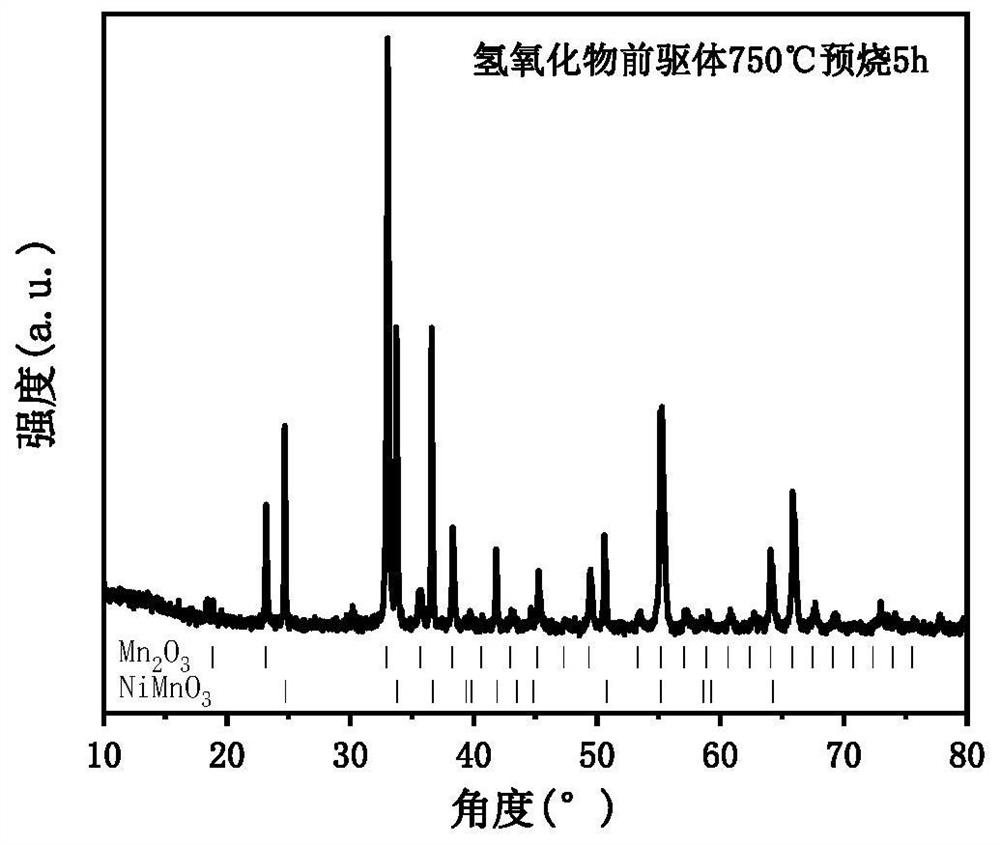
[0070] S3, putting the hydroxide precursor into a muffle furnace, and calcining at 750° C. for 5 hours to obtain an oxide precursor;
[0071] S4. Mix and stir the oxide precu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com