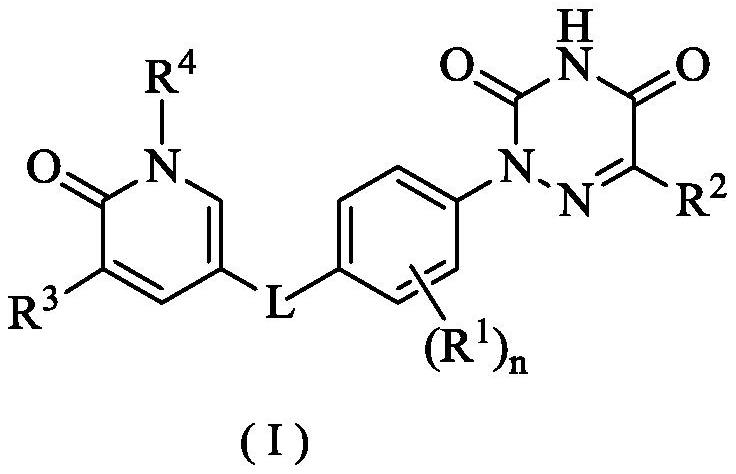
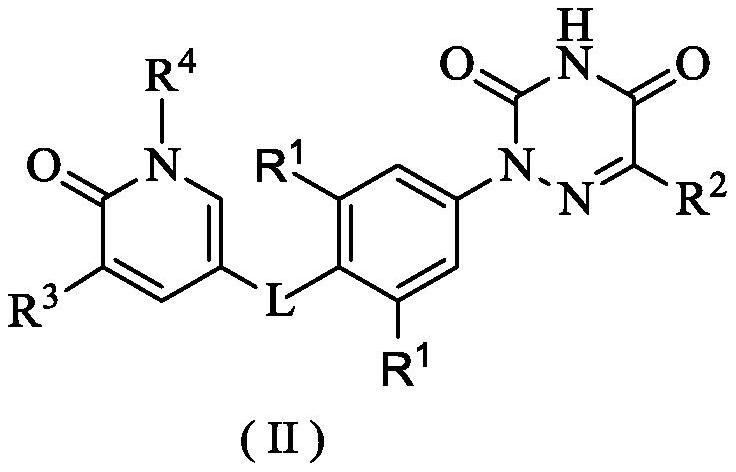
6-oxo-1, 6-dihydropyridine derivative, preparation method thereof and application of 6-oxo-1, 6-dihydropyridine derivative in medicine
A compound, alkoxy technology, applied in hyperlipidemia, diabetes, drugs, can solve the problem of not being able to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
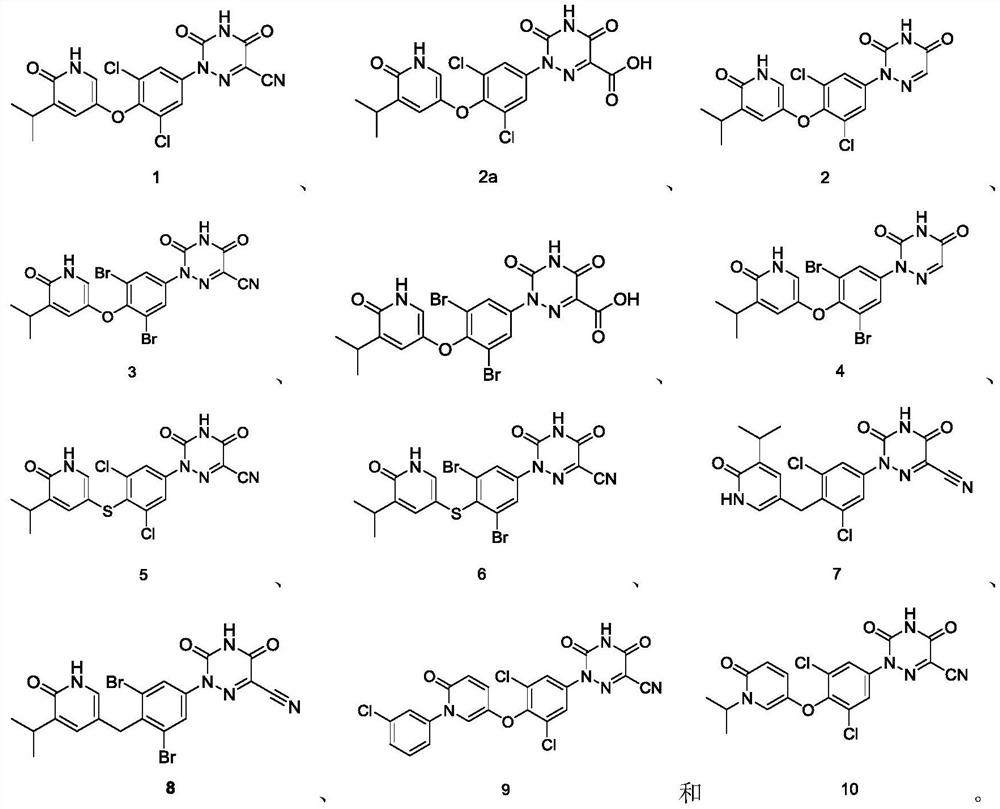
[0209] 2-(3,5-Dichloro-4-((5-isopropyl-6-oxo-1,6-dihydropyridin-3-yl)oxy)phenyl)-3,5-dioxo Generation-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile 1
[0210]
[0211] first step
[0212] 3-(prop-1-en-2-yl)pyridin-2(1H)-one 1c
[0213] 3-Bromopyridin-2(1H)-one 1a (1.40g, 8.04mmol, Shaoyuan Chemical Technology (Shanghai) Co., Ltd.), isopropenylboronic acid pinacol ester 1b (1.62g, 9.64mmol, Shaoyuan Chemical Technology (Shanghai) Co., Ltd.) was dissolved in N,N-dimethylformamide (20mL) and water (5mL), potassium phosphate (3.42g, 16.11mmol), tetrakistriphenylphosphine palladium (465mg, 0.40mmol ), stirred at 95°C for 3 hours. After filtration and concentration under reduced pressure to remove the solvent, the resulting residue was purified by silica gel column chromatography with eluent system B to obtain the title compound 1c (530 mg, yield: 48.7%).
[0214] MS m / z (ESI): 136.1 [M+1].
[0215] 1 H NMR (400MHz, CDCl 3 ):7.58(dd,1H),7.53(dd,1H),6.49(t,1H),5.66(s,1H)...
Embodiment 2
[0240] 2-(3,5-dichloro-4-((5-isopropyl-6-oxo-1,6-dihydropyridin-3-yl)oxy)phenyl)-1,2,4- Triazine-3,5(2H,4H)-dione 2
[0241]
[0242] first step
[0243] 2-(3,5-Dichloro-4-((5-isopropyl-6-oxo-1,6-dihydropyridin-3-yl)oxy)phenyl)-3,5-dioxo Dai-2,3,4,5-tetrahydro-1,2,4-triazine-6-carboxylic acid 2a
[0244] Compound 1 (86 mg, 0.20 mmol) was dissolved in acetic acid (6 mL), concentrated hydrochloric acid (1 mL), and stirred at 120° C. for 2 hours. The reaction of the raw materials was not complete, acetic acid (5 mL) and concentrated hydrochloric acid (1 mL) were added and the stirring was continued for 2 hours, and the reaction of the raw materials was complete. After concentration, the obtained residue was purified to obtain the title compound 2a (90 mg, yield: 100%).
[0245] MS m / z (ESI): 451.0 [M-1].
[0246] second step
[0247] 2-(3,5-dichloro-4-((5-isopropyl-6-oxo-1,6-dihydropyridin-3-yl)oxy)phenyl)-1,2,4- Triazine-3,5(2H,4H)-dione 2
[0248] Compound 2a (90 mg,...
Embodiment 3
[0252] 2-(3,5-Dibromo-4-((5-isopropyl-6-oxo-1,6-dihydropyridin-3-yl)oxy)phenyl)-3,5-dioxo Generation-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile 3
[0253]
[0254] first step
[0255] 5-(2,6-Dibromo-4-nitrophenoxy)-3-isopropylpyridin-2(1H)-one 3b
[0256] Compound 1e (100mg, 0.65mmol) was dissolved in N,N-dimethylformamide (8mL), and 1,3-dibromo-2-fluoro-5-nitrobenzene 3a (205mg, 0.69mmol) was added successively, Potassium carbonate (722mg, 5.22mmol), stirred at 55°C for 18 hours. After filtering, the filter cake was washed with a mixed solvent (20 mL×2) of dichloromethane and methanol (V / V=5 / 1), and acetic acid was slowly added dropwise until the pH of the filtrate was 5-6. Concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography with eluent system A to obtain the title compound 3b (150 mg, yield: 53.1%).
[0257] MS m / z (ESI): 432.9 [M+1].
[0258] second step
[0259] 5-(4-Amino-2,6-dibromophenoxy)-3-isop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com