Anti-IL-23R antibody and application thereof
An antibody and antigen technology, applied in the direction of antibodies, antibody medical components, applications, etc., can solve problems such as unsatisfactory properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1 Preparation of anti-IL-23 antibody mouse monoclonal antibody
[0064] 6-8 week old BALB / c mice (commercially purchased) were first immunized with 50 μg of commercial human IL-23R-ECD-Fc protein (Sino Biological, Cat. No. H02H). On the 14th day and 28th day after the first immunization, the immunized mice were immunized again with 25 μg IL-23R-ECD-Fc protein. The serum titer of immunized mice was detected by enzyme-linked immunosorbent assay (Elisa). The IL-23R-His (ACROBiosystem, H52H4) antigen was diluted to 0.5 μg / ml with PBS, and coated on a microwell plate overnight at 4°C. Then 1% BSA-PBS blocking solution was used to block at 37°C for 1h; after washing the plate with PBST, serum dilutions from mice were added to the plate and incubated at 37°C for 1hr. Wash the plate and add HRP-labeled goat anti-mouse IgG (Sigma, A0170) (1:10000), react at 37°C for 0.5h, wash the plate and add TMB solution, react at room temperature for 5 minutes in the dark, add 2...
Embodiment 2
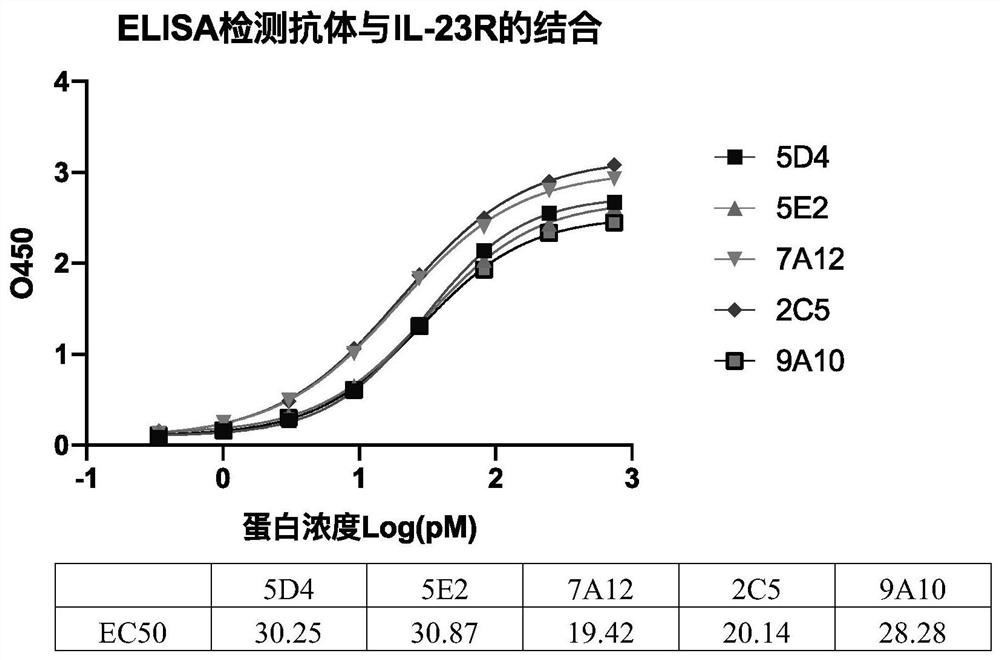
[0066] Example 2 Detection of binding of antibody to human IL-23R
[0067] The binding ability of the purified antibody to IL-23R protein was detected by Elisa method. The specific method was as described in Example 1, wherein the initial concentration of the mouse antibody was 740 pM, and a total of 10 gradients were obtained by 3-fold serial dilution with PBS. Each antibody was tested in duplicate wells, and the EC50 was analyzed by curve fitting. As a result, it was confirmed that the antibodies named mAb#5D4, mAb#5E2, mAb#7A12, mAb#2C5, and mAb#9A10 had high binding activity to human IL-23R ( figure 1 ).
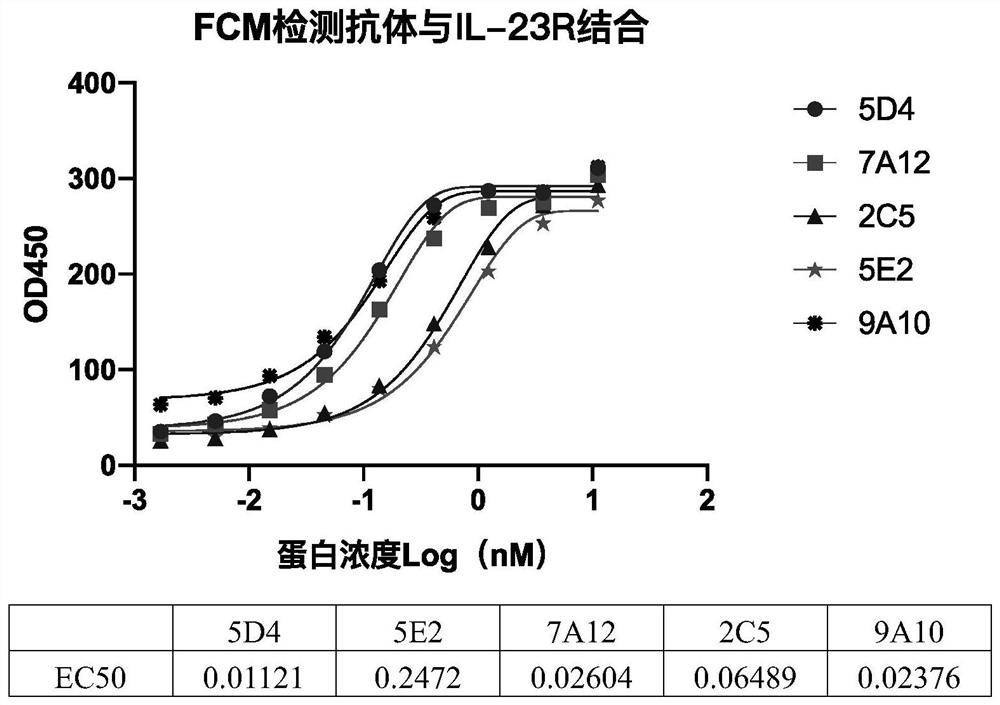
[0068] Flow cytometry (FCM) was used to analyze the binding of the above mouse monoclonal antibodies to IL-23R on the membrane of 293 cells 293-IL23 Res (Novoprotein, XCC05) that exogenously overexpressed IL-23R. The cells were reacted with different concentrations of monoclonal antibodies (the highest concentration was 11.11 nM, 3-fold serial dilution, a total of 9 co...
Embodiment 3
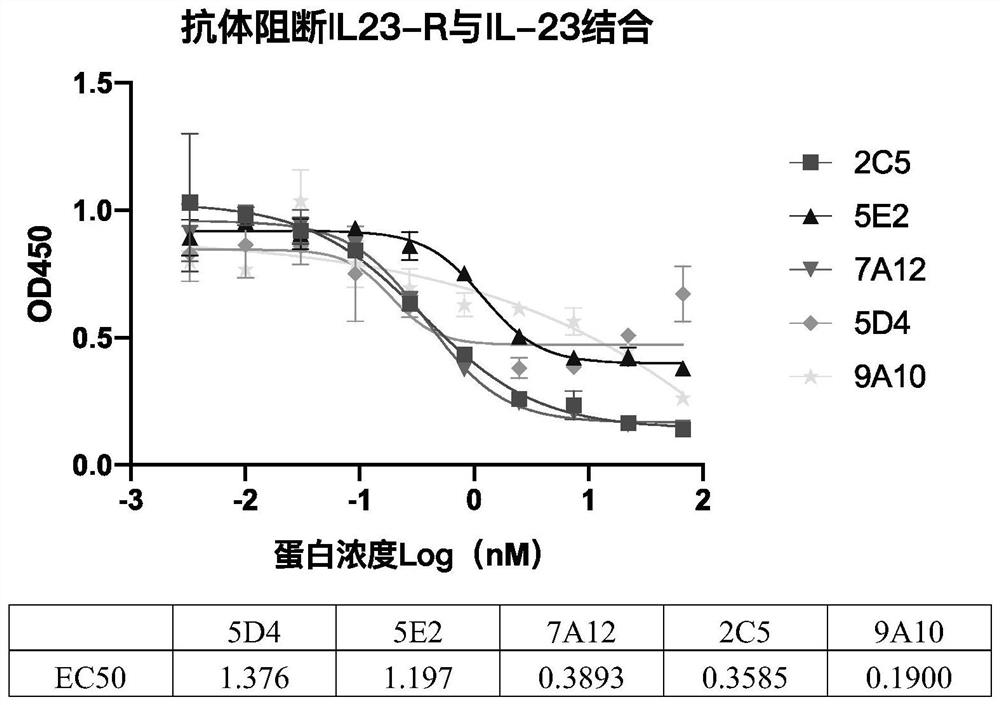
[0069] Example 3 Blocking Effect of Antibodies on the Binding of IL-23R and IL-23 Ligand
[0070] It was further tested whether these antibodies could block the binding of IL-23R and IL-23. IL-23R (ACROBiosystem, ILR-H5254) was diluted to 1 μg / mL, added to the microtiter plate at 100 μL / well, and coated overnight at 4°C. After washing the plate, 240 μL of 5% BSA-PBST was added to each well and blocked at 37°C for 1 hour. Dilute biotin-labeled IL-23α & IL-12β heterodimer protein (ACROBiosystem, ILB-H82W6) to 0.2μg / mL with diluent (1×PBST); dilute each antibody sample to 66.7nM, and then perform a 3-fold gradient Diluted to obtain a total of 10 concentration points. Add 50 μL of serially diluted antibody samples and 50 μL of IL-23α & IL-12β to each well, place at 37°C for 1 hour and wash the plate three times, add 100 μL of HRP-streptavidin (Abcam, Ab7403) to each well after dilution, and horizontally place at 37°C After standing for 1 hour, wash 3 times. Add 100 μL TMB chro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com