Synthesis method of pyrimidine aminoethyl methacrylate compound
The technology of pyrimidine aminoethyl methacrylate and isocyanoethyl methacrylate is applied in the field of synthesis of pyrimidine aminoethyl methacrylate compounds, and can solve the problems of environmental pollution, low yield and purity of finished products, and the like, Achieve the effect of ensuring purity, improving yield, and avoiding insufficient reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
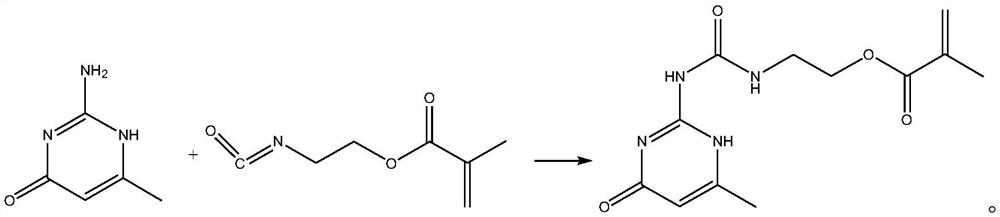
[0023] The synthetic method of pyrimidine aminoethyl methacrylate compound, its synthetic steps are as follows:
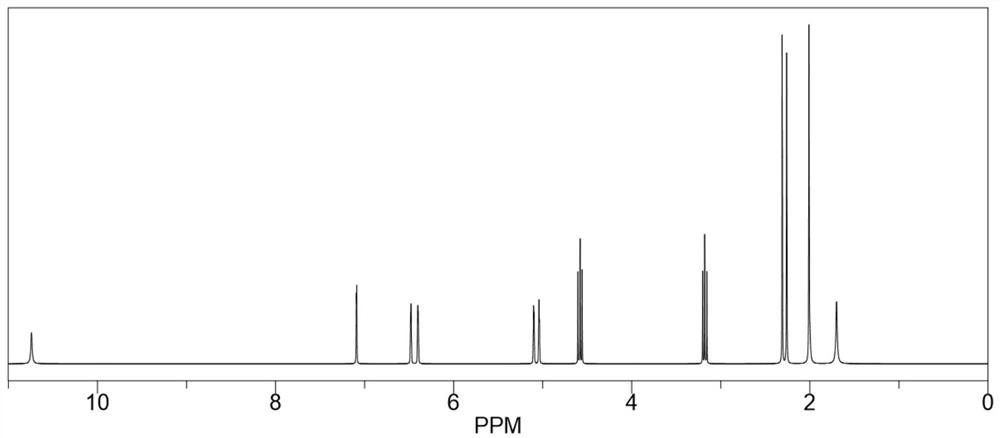
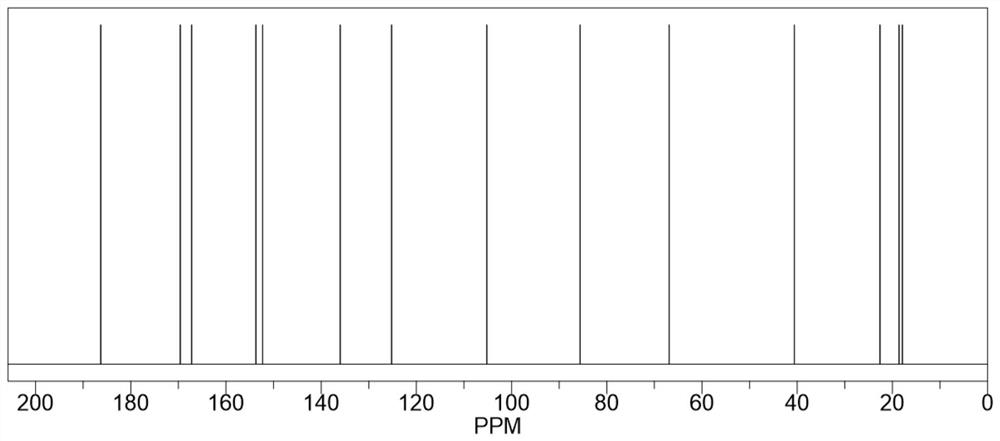
[0024] Take 40mL of dimethyl sulfoxide and add it to the reaction flask, heat to 135°C, add 8.0g (0.064mol) of 2-amino-4-hydroxy-6-methylpyrimidine and stir to dissolve, after complete dissolution, move to 25°C Rapidly cool down to 25°C in a water bath environment, then quickly add 11.0g (0.071mol) isocyanoethyl methacrylate, and maintain 25°C for addition reaction for 6 hours. During the reaction process, the reaction system is always maintained at 25°C, which can inhibit It polymerizes violently, producing by-products. After the addition reaction was completed, it was vacuum filtered and washed three times with excess acetone, and the resulting white solid was dried in vacuo to obtain 17.65 g of methacrylic acid 2-[[[(1,6-dihydro-4-methyl-6 -Oxo-2-pyrimidinyl)amino]carbonyl]amino]ethyl ester, yield 98.39%, purity 99.5%, specific chemical reaction formula is as f...
Embodiment 2
[0028] The synthetic method of pyrimidine aminoethyl methacrylate compound, its synthetic steps are as follows:
[0029] Take 64mL of dimethyl sulfoxide and add it to the reaction flask, heat to 130°C, add 8.0g (0.064mol) of 2-amino-4-hydroxy-6-methylpyrimidine and stir to dissolve, after complete dissolution, move to 20°C Rapidly cool down to 20°C in a water bath environment, then quickly add 12.9g (0.083mol) isocyanoethyl methacrylate, maintain 20°C for addition reaction for 8 hours, keep the reaction system at 20°C during the reaction process, which can inhibit It polymerizes violently, producing by-products. After the addition reaction was completed, it was vacuum filtered and washed three times with excess acetone, and the resulting white solid was dried in vacuo to obtain 17.68 g of methacrylic acid 2-[[[(1,6-dihydro-4-methyl-6 -Oxo-2-pyrimidinyl)amino]carbonyl]amino]ethyl ester, yield 98.56%, purity 99.6%.
Embodiment 3
[0031] The synthetic method of pyrimidine aminoethyl methacrylate compound, its synthetic steps are as follows:
[0032] Take 50mL of dimethyl sulfoxide and add it to the reaction flask, heat to 140°C, add 8.0g (0.064mol) of 2-amino-4-hydroxy-6-methylpyrimidine and stir to dissolve, after completely dissolved, move to 28°C Rapidly cool down to 28°C in a water bath environment, then quickly add 11.9g (0.077mol) isocyanoethyl methacrylate, and maintain 28°C for addition reaction for 5 hours. During the reaction, the reaction system is always maintained at 28°C, which can inhibit It polymerizes violently, producing by-products. After the addition reaction was completed, it was vacuum filtered and washed three times with excess acetone, and the resulting white solid was dried in vacuo to obtain 17.58 g of methacrylic acid 2-[[[(1,6-dihydro-4-methyl-6 -Oxo-2-pyrimidinyl)amino]carbonyl]amino]ethyl ester, yield 98.00%, purity 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com