New application of artesunate and composition containing artesunate
A kind of technology of artesunate and composition, applied in the new application field of composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
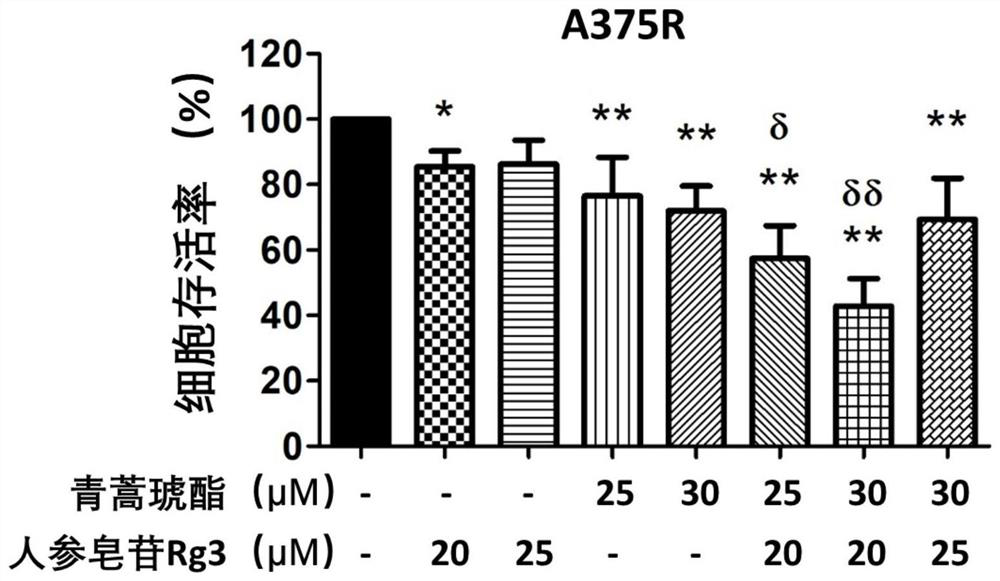
[0047] Example 1. Effects of ginsenoside Rg3 and artesunate alone and in combination on the proliferation of melanoma cells resistant to BRAF (V600E) targeting drugs
[0048] 1.1. Experimental materials
[0049] (1) Ginsenoside 20-R-Rg3: purchased from Nanjing Yuanzhi Biotechnology Co., Ltd.; weigh a certain amount of ginsenoside 20-R-Rg3, and dissolve it into 20 mM Rg3 solution (mother liquor) with dimethyl sulfoxide (DMSO) ), subpackage, and store in a -20°C refrigerator for later use.
[0050] (2) Artesunate: purchased from Chengdu Mansite Biotechnology Co., Ltd.; weigh a certain amount of artesunate, dissolve it in DMSO to 50mM artesunate solution (mother solution), subpackage, and store at -20°C Refrigerator for spare.
[0051] (3) Vemurafenib: produced by LC Laboratories; a certain amount of vemurafenib was weighed, dissolved in DMSO to form a 10 mM vemurafenib solution (mother solution), subpackaged, and stored in a -20°C refrigerator for later use.
[0052] (4) A375...
Embodiment 2
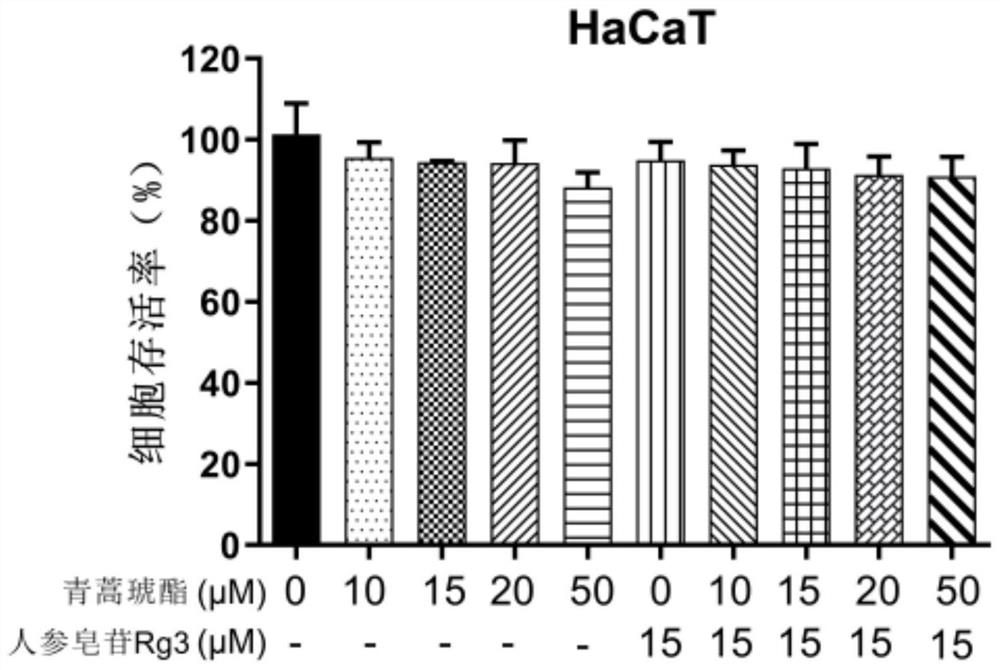
[0081] Example 2. Effects of ginsenoside Rg3 and artesunate alone and in combination on normal cell survival
[0082] 2.1. Experimental materials
[0083] (1) Ginsenoside 20-R-Rg3: purchased from Nanjing Yuanzhi Biotechnology Co., Ltd.; weigh a certain amount of ginsenoside Rg3, dissolve it in DMSO into a 20mM Rg3 solution (mother solution), subpackage it, and store it in a -20°C refrigerator for later use .
[0084] (2) Artesunate: purchased from Chengdu Mansite Biotechnology Co., Ltd.; weigh a certain amount of artesunate, dissolve it in DMSO to 50mM artesunate solution (mother solution), subpackage, and store at -20°C Refrigerator for spare.
[0085] (3) Normal cell line: human immortalized keratinocyte HaCaT; purchased from ATCC cell bank in the United States and stored in liquid nitrogen.
[0086] (4) DMEM medium powder: produced by Gibco, USA.
[0087] (5) 0.5% trypsin-EDTA (10X) (Trypsin): 100 mL / bottle, produced by Gibco, USA.
[0088] (6) Fetal bovine serum: 500 ...
Embodiment 3
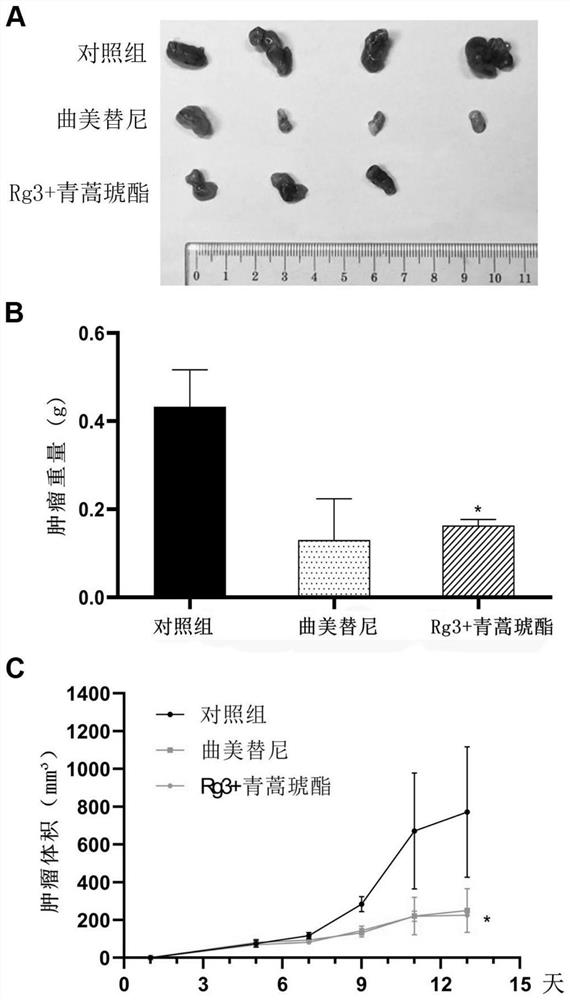
[0106] Example 3, Effects of Ginsenoside Rg3 Combined with Artesunate on Melanoma Mice Bearing Resistance to BRAF (V600E) Inhibitors
[0107] 3.1. Experimental materials
[0108] Experimental animals: male BALB / c-nu / nu nude mice (about 8 weeks old), provided by the Animal Experiment Center of the Chinese University of Hong Kong. They were raised in a sterile environment with constant temperature and pressure in the Experimental Animal Center of Hong Kong Baptist University.
[0109] Cells A375R with acquired resistance to vemurafenib: A375 cells were purchased from the ATCC cell bank in the United States. Melanoma cells A375R resistant to vemurafenib were established in Example 1, and subsequently treated with 2 μM vemurafenib and The cells were cultured in DMEM with 10% FBS to maintain their drug resistance.
[0110] Ginsenoside Rg3 intragastric solution: Weigh 7.2mg ginsenoside Rg3 and dissolve it in 0.3mL of DMSO, then dilute it to 6mL with carboxymethylcellulose sodium (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com