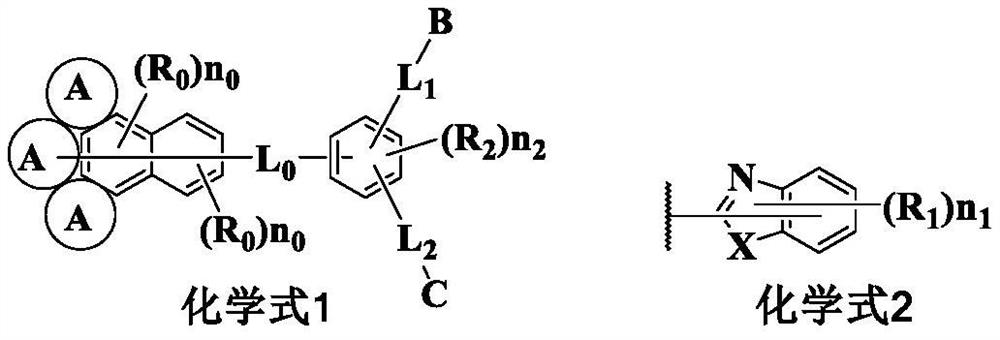
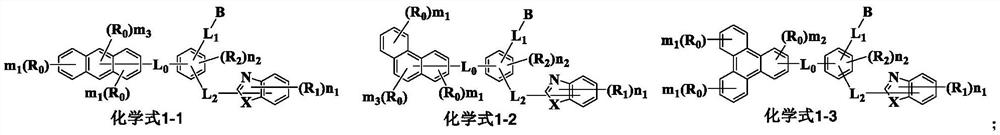
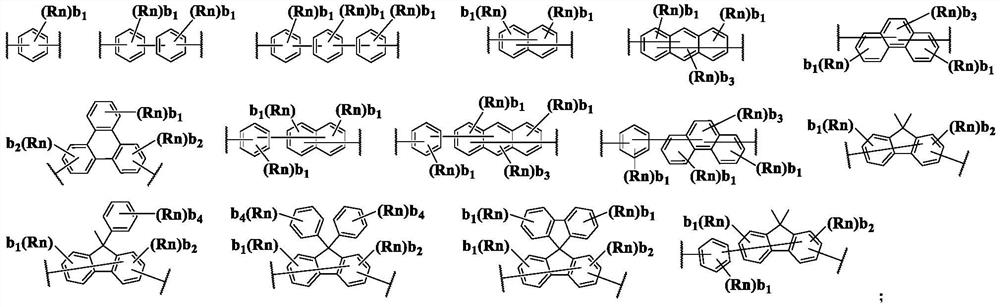
Compound containing condensed aryl group and organic electroluminescent device thereof
A compound and aryl technology, applied in the field of optoelectronic materials, can solve the problems of holes and electrons that cannot be combined, have no hole blocking effect, and unbalanced carrier injection, so as to improve the luminous efficiency, improve the light extraction efficiency, The effect of improving luminous efficiency and lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0120] Preparation of intermediate 1-1:
[0121] Under nitrogen protection, a-1 (302.60mmol, 92.96g), b-1 (300.00mmol, 46.91g), Pd(PPh 3 ) 4 (2.30mmol, 2.66g) and 900mL of toluene, 300mL of ethanol were added to the reaction flask, the mixture was stirred, followed by 300mL of 2M K 2 CO 3 The aqueous solution was injected into the above solution through a syringe, and the reaction was refluxed for 2 hours. After the reaction was completed and cooled down to room temperature, the filter cake was obtained by suction filtration, and the filter cake was rinsed with ethanol. Finally, the filter cake was recrystallized with toluene / ethanol=10:3 to obtain intermediate 1-1 (83.35 g, yield 82%); HPLC purity ≥ 99.46%. Mass Spectrum m / z: 338.0873 (Theoretical: 338.0862).
[0122] Preparation of intermediate 1-2:
[0123] Under nitrogen protection, intermediate 1-1 (220.00 mmol, 74.54 g), d-1 (230.00 mmol, 58.41 g) and KOAc (450.00 mmol, 44.16 g) were dissolved in anhydrous dioxane ...
Embodiment 33
[0217] [Example 33] triplet energy level test
[0218] Test samples: the compounds prepared in the synthesis examples of the present invention and comparative compounds 1-3.
[0219] Test instrument: fluorescence spectrophotometer (Hitachi F-4600).
[0220] Test conditions: Toluene is the solvent, the concentration is 2×10 -5 mol / L, the temperature is -78°C. The triplet energy level (T 1 ), the calculation results are shown in Table 1:
Embodiment 34
[0221] [Example 34] Glass transition temperature test
[0222] Test samples: compounds prepared in the synthesis examples of the present invention and comparative compounds 1-4.
[0223] Test instrument: DSC 25 differential scanning calorimeter (TA company in the United States);
[0224] Test conditions: The test atmosphere is nitrogen, the flow rate of nitrogen is 50mL / min; the heating rate is 10°C / min, and the temperature range is 50-350°C. The glass transition temperature (Tg) test results are shown in Table 1:
[0225] Table 1:
[0226]
[0227] As can be seen from Table 1, compared with comparative compound 1, comparative compound 2 and comparative compound 4, the compound of the present invention has a higher glass transition temperature, and the thermal stability and film-forming property of the material are improved. In the electroluminescent device, the service life of the device can be extended; on the other hand, compared with the comparative compounds 1-3, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com