Environment-responsive hyaluronic acid-podophyllotoxin prodrug micelle as well as preparation method and application thereof
A technology of hyaluronic acid and podophyllotoxin, which can be used in pharmaceutical formulations, antineoplastic drugs, drug combinations, etc., can solve problems such as poor water solubility, toxic side effects, and non-specific accumulation, and achieve good blood compatibility and broad application prospects , good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
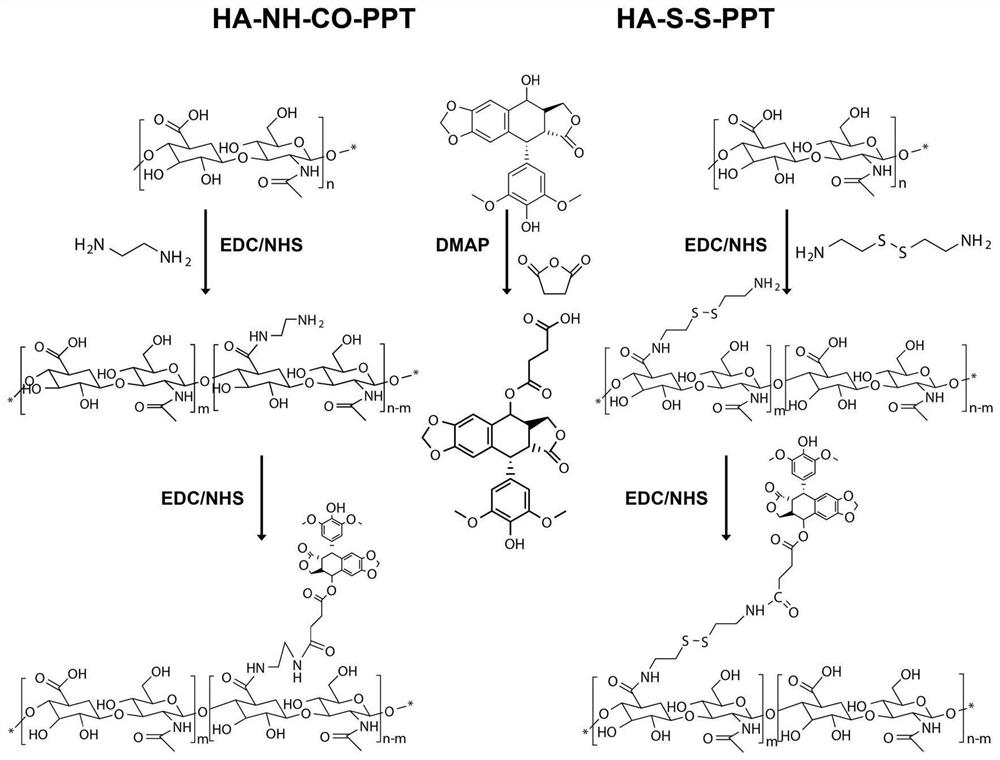
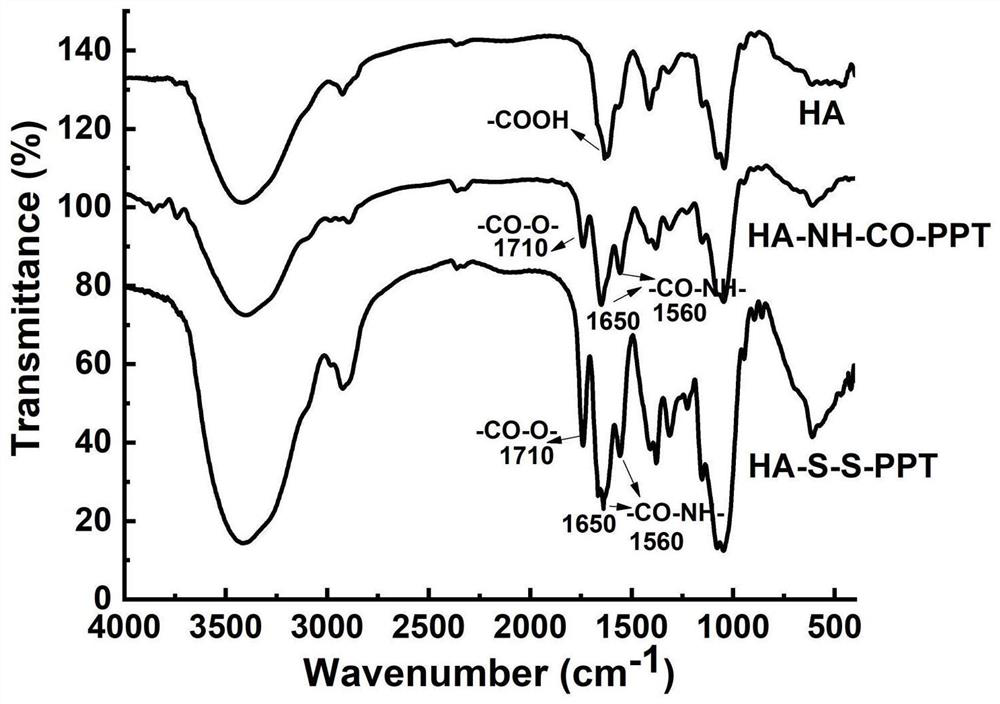
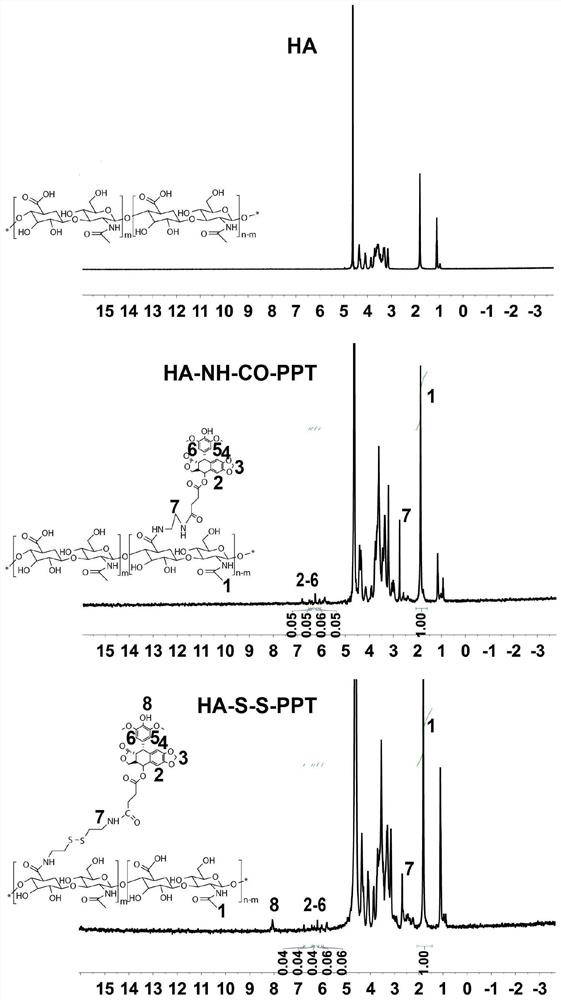
[0052] (1) HA-NH 2 Preparation: Hyaluronic acid (HA, 400 mg, equivalent to 1054 μmol of COOH groups) with a molecular weight of 3000-10000 Da, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were dissolved in a PBS solution (5 mL) with a pH of 8.0 at a molar ratio of 1:1:1, and reacted in an ice bath for 1 hour to activate the carboxyl group of HA to obtain a solution 1. Then, ethylenediamine and HA in an equimolar amount were dissolved in 5 mL of dimethyl sulfoxide (DMSO) and added to the above solution 1, and reacted at 25° C. for 24 hours. After the reaction, the resulting mixture was dialyzed against deionized water (MWCO 2000) for 72 hours, and the deionized water was changed every 6 hours. Finally, the solution was filtered and lyophilized to obtain HA-NH 2 .
[0053] (2) Preparation of PPT-COOH: Dissolve succinic anhydride (SA, 1.5mmol), podophyllotoxin (PPT) and 4-dimethylaminopyridine (DMAP) in a molar ratio of 15:1...
Embodiment 2
[0057] (1) HA-NH 2 Preparation: Hyaluronic acid (HA, 400 mg, equivalent to 1054 μmol of COOH groups) with a molecular weight of 3000-10000 Da, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were dissolved in a PBS solution (5 mL) with a pH of 8.0 at a molar ratio of 1:1:1, and reacted in an ice bath for 1 hour to activate the carboxyl group of HA to obtain a solution 1. Then, ethylenediamine and HA in an equimolar amount were dissolved in 5 mL of dimethyl sulfoxide (DMSO) and added to the above solution 1, and reacted at 25° C. for 24 hours. After the reaction, the resulting mixture was dialyzed against deionized water (MWCO 2000) for 72 hours, and the deionized water was changed every 6 hours. Finally, the solution was filtered and lyophilized to obtain HA-NH 2 .
[0058] (2) Preparation of PPT-COOH: Dissolve succinic anhydride (SA, 1.5mmol), podophyllotoxin (PPT) and 4-dimethylaminopyridine (DMAP) in a molar ratio of 15:1...
Embodiment 3
[0062] (1) HA-NH 2 Preparation: Hyaluronic acid (HA, 400 mg, equivalent to 1054 μmol of COOH groups) with a molecular weight of 3000-10000 Da, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were dissolved in a PBS solution (5 mL) with a pH of 8.0 at a molar ratio of 1:1:1, and reacted in an ice bath for 1 hour to activate the carboxyl group of HA to obtain a solution 1. Then, ethylenediamine and HA in an equimolar amount were dissolved in 5 mL of dimethyl sulfoxide (DMSO) and added to the above solution 1, and reacted at 25° C. for 24 hours. After the reaction, the resulting mixture was dialyzed against deionized water (MWCO 2000) for 72 hours, and the deionized water was changed every 6 hours. Finally, the solution was filtered and lyophilized to obtain HA-NH 2 .
[0063] (2) Preparation of PPT-COOH: Dissolve succinic anhydride (SA, 1.5mmol), podophyllotoxin (PPT) and 4-dimethylaminopyridine (DMAP) in a molar ratio of 15:1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com