Preparation method of sildenafil intermediate
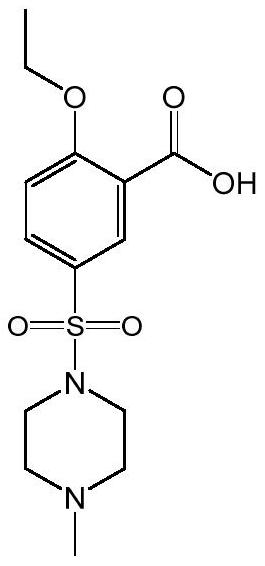
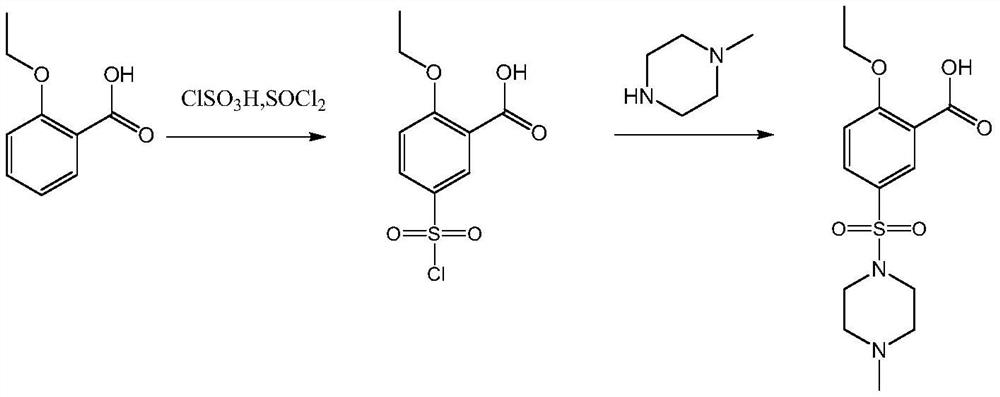
A technology of sildenafil and intermediates, which is applied in the field of preparation of sildenafil intermediate 2-ethoxy-5-benzoic acid, can solve the problems of equipment corrosion, low yield, unfriendly environment and the like, and achieves the Improve production efficiency, reduce production costs, mature and stable processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
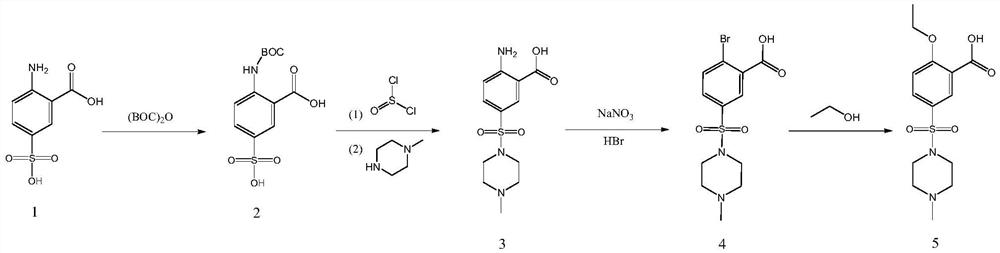
[0046] 1) Synthesis of 2-N(BOC)-5-sulfonic acid benzoic acid (compound of formula 2)
[0047] Add 50g of 2-amino-5-sulfonic acid benzoic acid into 250ml of dichloromethane, add 55.27g of di-tert-butyl dicarbonate dropwise under temperature control at 20-30°C, then react at 20-30°C for 2 hours, and pass through 250g Wash twice with water to obtain a dichloromethane solution of 2-(N-BOC)-5-sulfobenzoic acid, control the temperature T≤35°C, distill under reduced pressure to dryness, and then add 250ml of dichloromethane, and detect KF≤0.1 %.
[0048] 2) Synthesis of 2-amino-5-(4-methylpiperazin-1-ylsulfonyl)benzoic acid (compound of formula 3)
[0049] (a) Control the temperature at 0-10°C, add 82.2g of thionyl chloride dropwise into the dichloromethane solution of the compound of formula 2, keep the temperature at 0-10°C for 6 hours, and slowly add 500g of water at 0-10°C after the reaction is completed Quenched in a mixed system composed of 50 g of sodium bicarbonate to pH 7-...
Embodiment 2
[0056] 1) Synthesis of 2-N(BOC)-5-sulfonic acid benzoic acid (compound of formula 2)
[0057] Add 50g of 2-amino-5-sulfonic acid benzoic acid into 250ml of dichloromethane, add 55.2g of di-tert-butyl dicarbonate dropwise under temperature control at 20-30°C, then react at 20-30°C for 2 hours, and pass through 250g Wash twice with water to obtain a dichloromethane solution of 2-(N-BOC)-5-sulfobenzoic acid, control the temperature T≤35°C, distill under reduced pressure to dryness, and then add 250ml of dichloromethane, and detect KF≤0.1 %.
[0058] 2) Synthesis of 2-amino-5-(4-methylpiperazin-1-ylsulfonyl)benzoic acid (compound of formula 3)
[0059] (a) Control the temperature at 0-10°C, add 82.1g of thionyl chloride dropwise into the dichloromethane solution of the compound of formula 2, keep the temperature at 0-10°C for 6h, and slowly add 500g of water at 0-10°C after the reaction is completed Quenched in a mixed system composed of 50.1 g of sodium bicarbonate to pH 7-8, a...
Embodiment 3
[0066] 1) Synthesis of 2-N(BOC)-5-sulfonic acid benzoic acid (compound of formula 2)
[0067] Add 50g of 2-amino-5-sulfonic acid benzoic acid into 250ml of dichloromethane, add 55.10g of di-tert-butyl dicarbonate dropwise at a temperature of 20-30°C, and then react at 20-30°C for 2 hours. Wash twice with water to obtain a dichloromethane solution of 2-(N-BOC)-5-sulfobenzoic acid, control the temperature T≤35°C, distill under reduced pressure to dryness, and then add 250ml of dichloromethane, and detect KF≤0.1 %.
[0068] 2) Synthesis of 2-amino-5-(4-methylpiperazin-1-ylsulfonyl)benzoic acid (compound of formula 3)
[0069] (a) Control the temperature at 0-10°C, add 82.3g of thionyl chloride dropwise into the dichloromethane solution of the compound of formula 2, keep the temperature at 0-10°C for 6 hours, and slowly add 500g of water at 0-10°C after the reaction is completed Quenched in a mixed system composed of 50 g of sodium bicarbonate to pH 7-8, and washed with water to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com