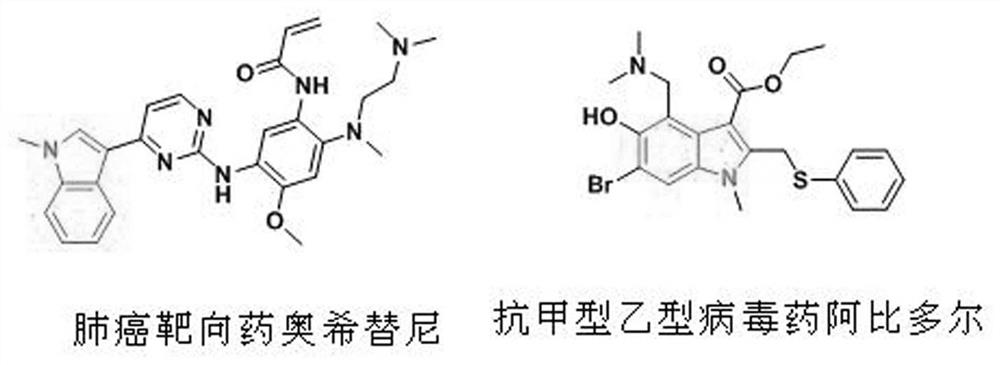
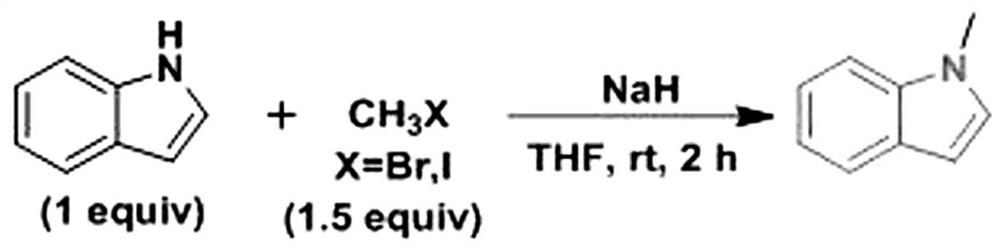
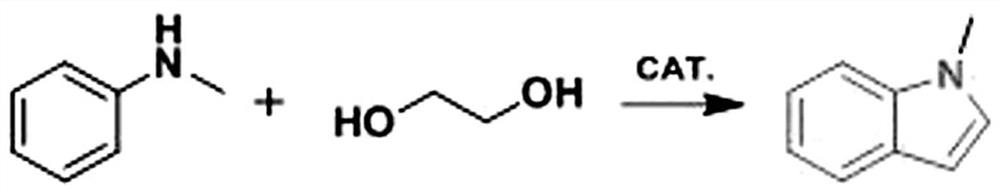
Synthesis method of N-methylindole
A technology of methyl indole and synthesis method, applied in the field of synthesis of N-methyl indole, capable of solving problems such as high temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] In order to make the purpose, technical solutions and advantages of the embodiments of the present invention clearer, the technical solutions in the embodiments of the present invention will be clearly and completely described below. Obviously, the described embodiments are part of the embodiments of the present invention, rather than All the embodiments; based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts all belong to the protection scope of the present invention.
[0021] Add 0.15 mmol of palladium-based (based on palladium content) catalyst, 0.45 mmol of co-catalyst a, and 0.45 mmol of co-catalyst b to a 25 mL pressure-resistant test tube, and then measure 54 mmol (3 mL) of ethylene glycol with a micro-sampler Add to the reaction tube, and then measure 1 mmol (108 μL) N - Methylaniline was added to the reaction tube. The reaction tube was placed in an oil bath at a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com