Recrystallization method of mirabegron alpha crystal form raw material
A mirabegron and recrystallization technology, applied in the field of medicine and chemical industry, can solve the problems of cumbersome operation, difficult to remove, increase material cost, etc., and achieve the effect of easy recovery of solvent, favorable for industrial production, and reduction of production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0050] Preparation Example: Preparation of Crude Mirabegron
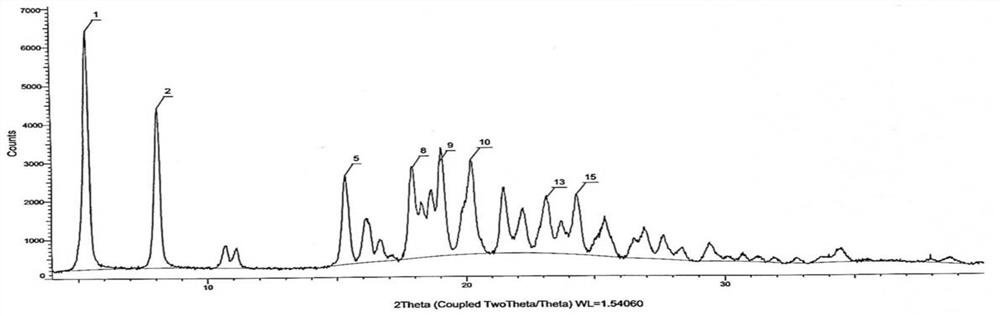
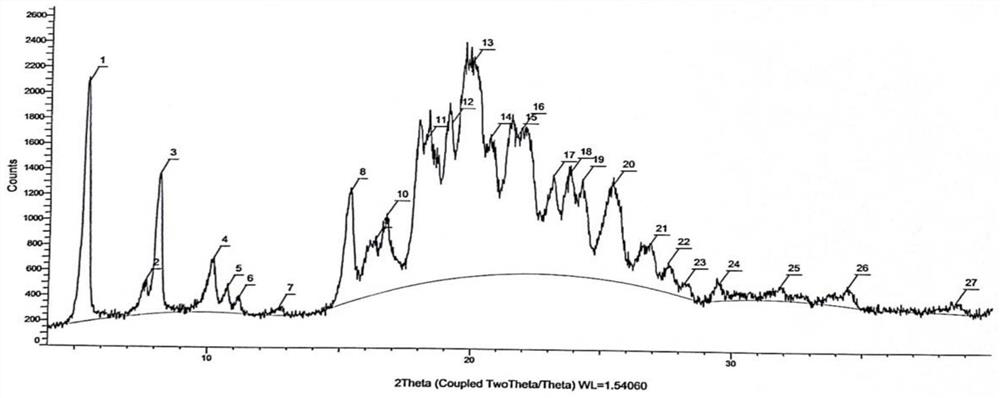
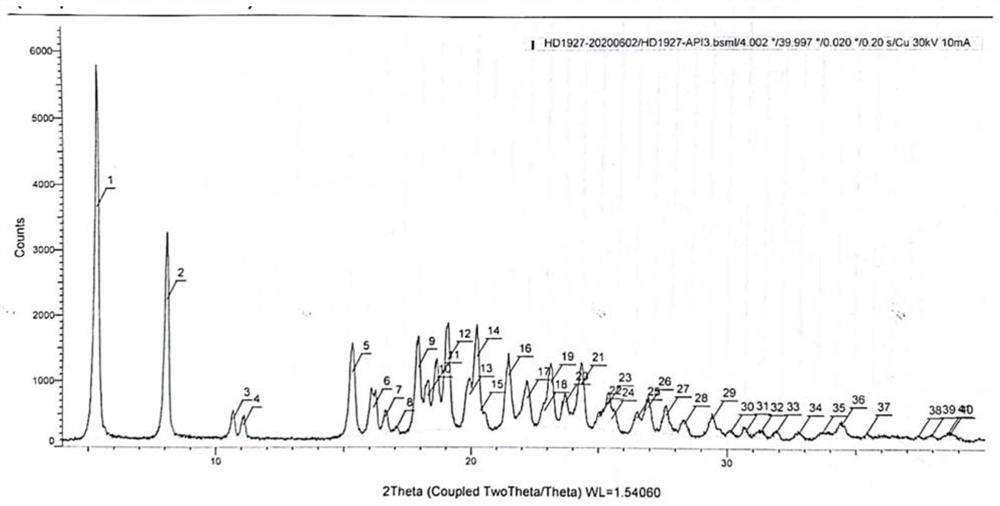
[0051] In a 50L reaction tank, add 15L of purified water, start stirring, and add 1Kg of (R)-2-{[2-(4-aminophenyl)ethyl]amino}-1-phenylethanol hydrochloride (Mira Veron intermediate), 0.67Kg 2-(2-aminothiazol-4-yl) acetic acid hydrochloride and 0.9Kg 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride Salt (EDCI), after adding, react at room temperature for 5 hours, TLC monitors that the reaction of the Mirabegron intermediate is complete, slowly add 7L of 5% sodium hydroxide aqueous solution that has been prepared in advance, and a white solid is precipitated during the feeding process, and the addition is completed , suction filtration, obtain wet product (purity 98.79%, impurity A content 0.19%, the retention time of impurity A is 39.8min), the purity detection result sees Figure 4 .
[0052] Add 8Kg ethanol and 14Kg water in the reaction tank, add wet product under stirring, be warming up to fully dis...
Embodiment 1-2、 comparative example 1-3
[0053] Embodiment 1-2, comparative example 1-3: the influence of solvent, cooling program and seed crystal on test
[0054] Add solvent into a 1000mL three-neck flask, add 30g of crude product under stirring, heat up and down program and add α-crystal seed (see Table 1 for details), filter with suction, and dry in vacuum at 70°C to obtain the raw material product of Mirabegron.
[0055] Table 1 The effect of cooling program and seed crystal on the test
[0056]
[0057]
[0058] It can be seen from the above table that when using a mixed solvent of water and ethanol for recrystallization, it is necessary to strictly control the heating and cooling program, which takes a long time and needs to add seed crystals at the same time, otherwise the pure target α crystal form raw material cannot be obtained, and When ethanol single solvent is used for recrystallization, the heating and cooling procedure is simple, the time consumption is greatly reduced, and the target α crystal...
Embodiment 2-11
[0059] Example 2-11: Investigation of different types of single solvents and activated carbon
[0060] Add a single solvent in the there-necked flask, add 30g of Mirabegron crude product under stirring, heat up to reflux, after fully dissolving, add activated carbon (see Table 2 for details), filter while hot, and quickly cool down to room temperature in a cold water bath. Suction filtration for about 0.5h, and vacuum drying at 70°C to obtain the raw material of Mirabegron α crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com