Lithium electrolyte of solvent self-sacrifice in-situ protection electrode, preparation and application thereof
A technology for protecting electrodes and electrolytes, applied in electrolytes, non-aqueous electrolytes, non-aqueous electrolyte batteries, etc., can solve problems such as poor cycle stability of high-voltage electrochemical systems, reduce electrolyte consumption, increase specific energy, and increase specific energy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
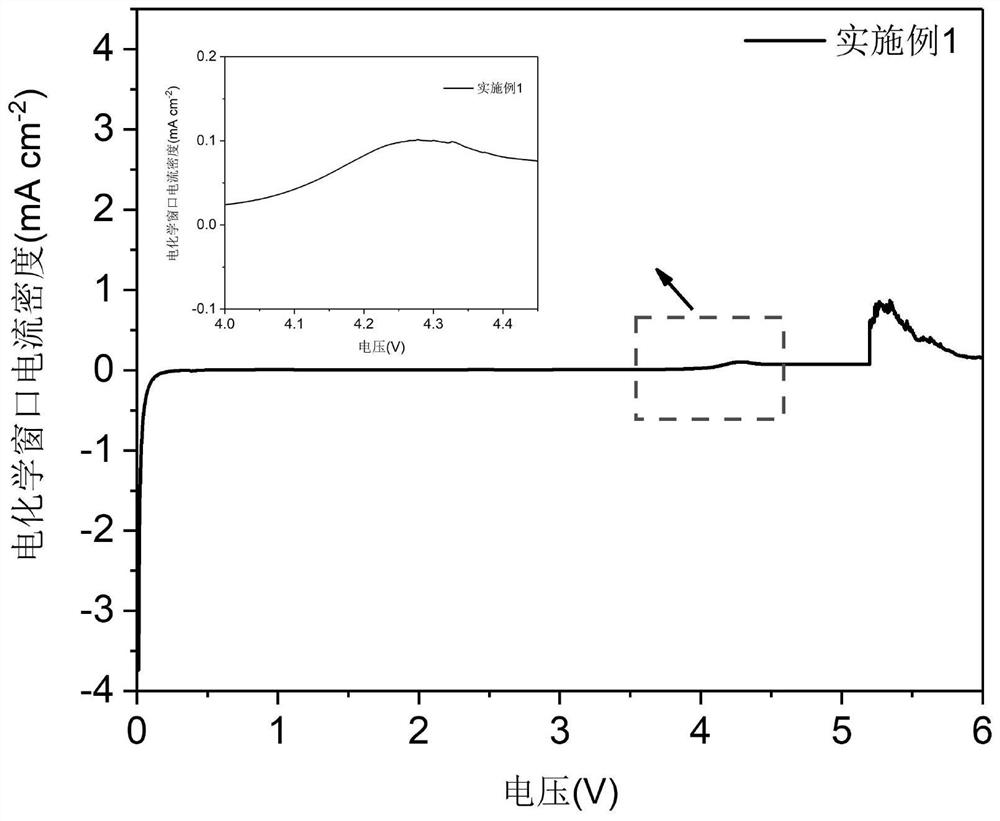
Embodiment 1
[0058] A preparation method of a lithium-based electrolyte in which a solvent self-sacrifices an in-situ protection electrode is as follows:
[0059] ① Solvent mixing: Weigh DMC and MMA as the main solvent according to the ratio in Table 1, and put FEC and TFEB as the fluorinated solvent into a stainless steel tank container, place the container in a propeller stirrer and stir at 400r / min for 1min to obtain the solvent A; where DMC:MMA=1:2 by volume ratio, FEC:TFEB=1:4;
[0060] ② Lithium salt mixing: weigh LATP and LiPF according to the ratio in Table 1 6 Lithium salt, in the state of stirring vortex in the solvent A described in step ①, add lithium salt to the vortex, then seal the container and transfer it to an ultrasonic device in an environment with a relative humidity of 0.5%, ultrasonic treatment for 30min to obtain Solvent A of lithium salt; by mass ratio LATP: LiPF 6 =1:23
[0061] ③ Self-sacrifice inducer mixing: Weigh AIBN and TTE as the self-sacrifice inducer a...
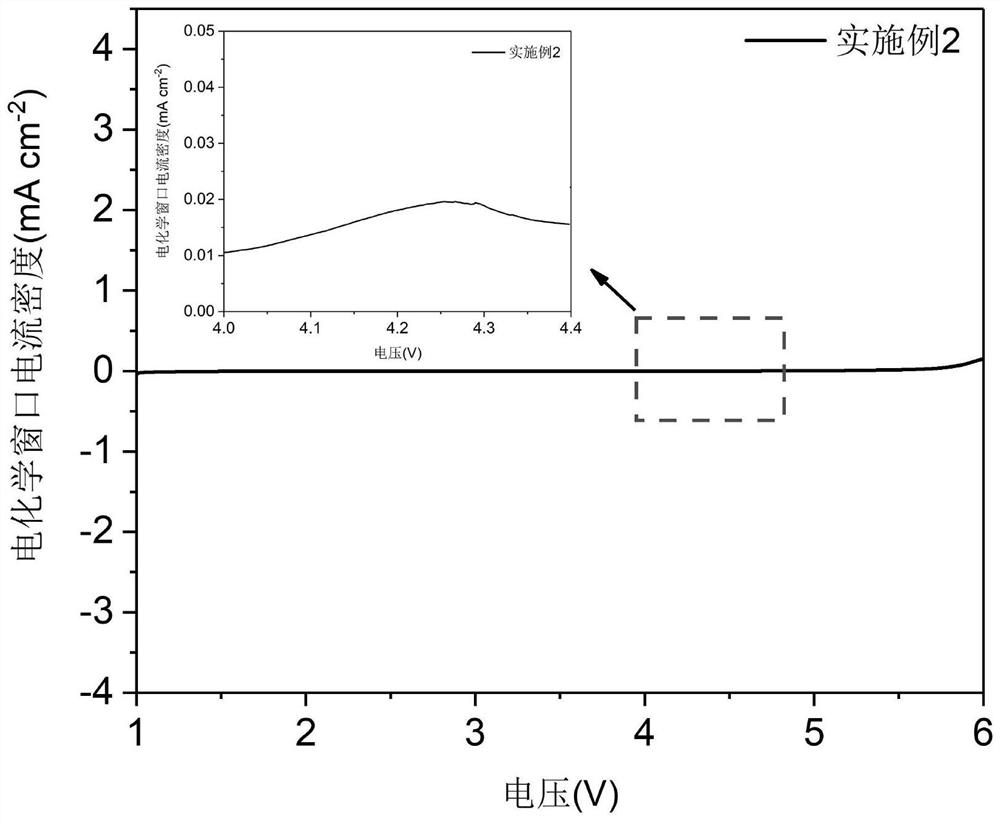
Embodiment 2
[0066] A preparation method of a lithium-based electrolyte in which a solvent self-sacrifices an in-situ protection electrode is as follows:
[0067] ①Solvent mixing: Weigh EMC and EA as the main solvent according to the ratio in Table 1, and put TFEB and TFPC as the fluorinated solvent into a polytetrafluoroethylene tank container, place the container in a magnetic stirrer and stir at 500r / min for 1min to obtain Solvent A;
[0068] ② Lithium salt mixing: weigh LLZTO, LiDOFB, LiPF according to the ratio in Table 1 6 Lithium salt, in the state of stirring vortex in the solvent A described in step ①, add lithium salt to the vortex, then seal the container and transfer it to an ultrasonic device in an environment with a relative humidity of 1.0%, and ultrasonically treat it for 30 minutes to obtain Solvent A of lithium salt;
[0069] ③ Self-sacrifice inducer mixing: Weigh VC and BPO as the self-sacrifice inducer according to the ratio in Table 1, and add them to the solvent A c...
Embodiment 3
[0074] A preparation method of a lithium-based electrolyte in which a solvent self-sacrifices an in-situ protection electrode is as follows:
[0075] ①Solvent mixing: Weigh EMC and EA as the main solvent according to the ratio in Table 1, and put TFEB and TFPC as the fluorinated solvent into a corundum tank container, place the container in a turbo stirrer and stir at 600r / min for 1min to obtain solvent A ;
[0076] ② Lithium salt mixing: weigh LiDOFB and LiBF according to the ratio in Table 1 4 Lithium salt, in the state of stirring vortex in the solvent A described in step ①, add lithium salt to the vortex, then seal the container and transfer it to an ultrasonic device in an environment with a relative humidity of 1.0%, and ultrasonically treat it for 10 minutes to obtain Solvent A of lithium salt;
[0077] ③ Self-sacrifice inducer mixing: weigh SL and MF according to the ratio in Table 1 3 As a self-sacrificing inducer, add it to the lithium salt-containing solvent A ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com