Fluorescent probe compound, and preparation method and application thereof
A fluorescent probe and compound technology, applied in the field of biological analysis, can solve the problems of cumbersome ligand operations and low accuracy, and achieve the effects of clear binding sites, convenient and fast detection, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
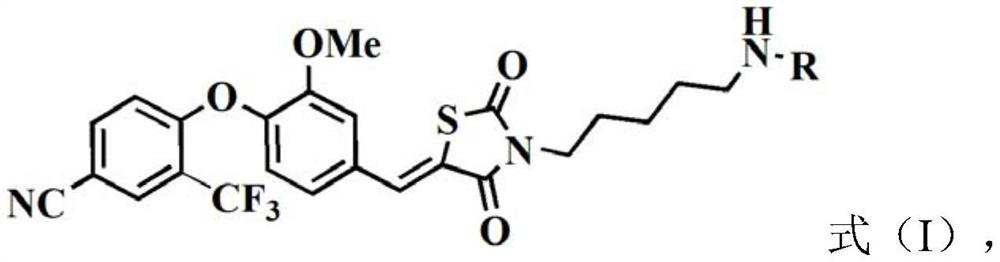
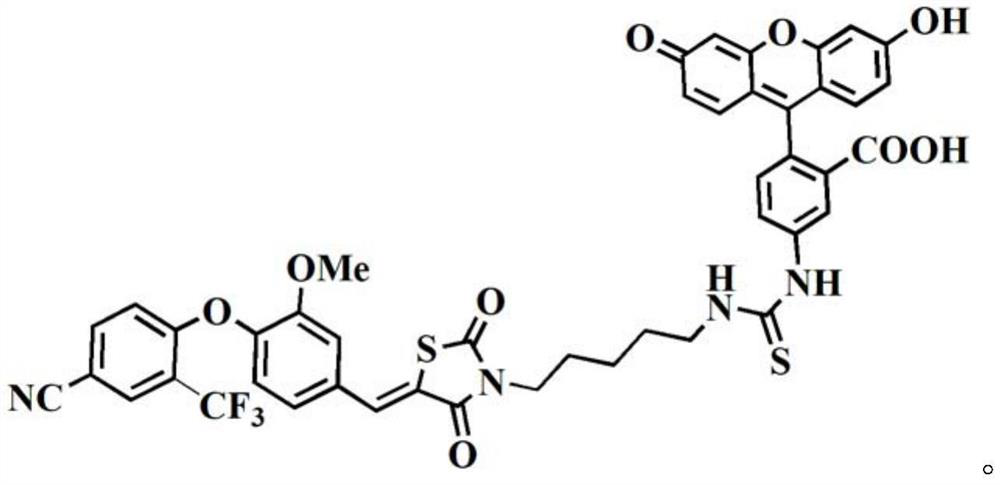
[0063] A second aspect of the present invention provides a method for preparing a fluorescent probe compound as shown in formula (I), comprising the following steps:
[0064] Under the condition that reaction solvent I and catalyst I exist, the compound shown in formula (II) is reacted I with the organic fluorescent molecule that has reactivity with amino group,
[0065]
[0066] According to the present invention, the compound represented by the formula (II) can provide a ligand that specifically interacts with the ERRα protein, and different organic fluorescent molecules with reactivity with the amino group can be selected according to application requirements, so as to be compatible with the formula (II) The compounds shown form the corresponding fluorescent probe compounds.
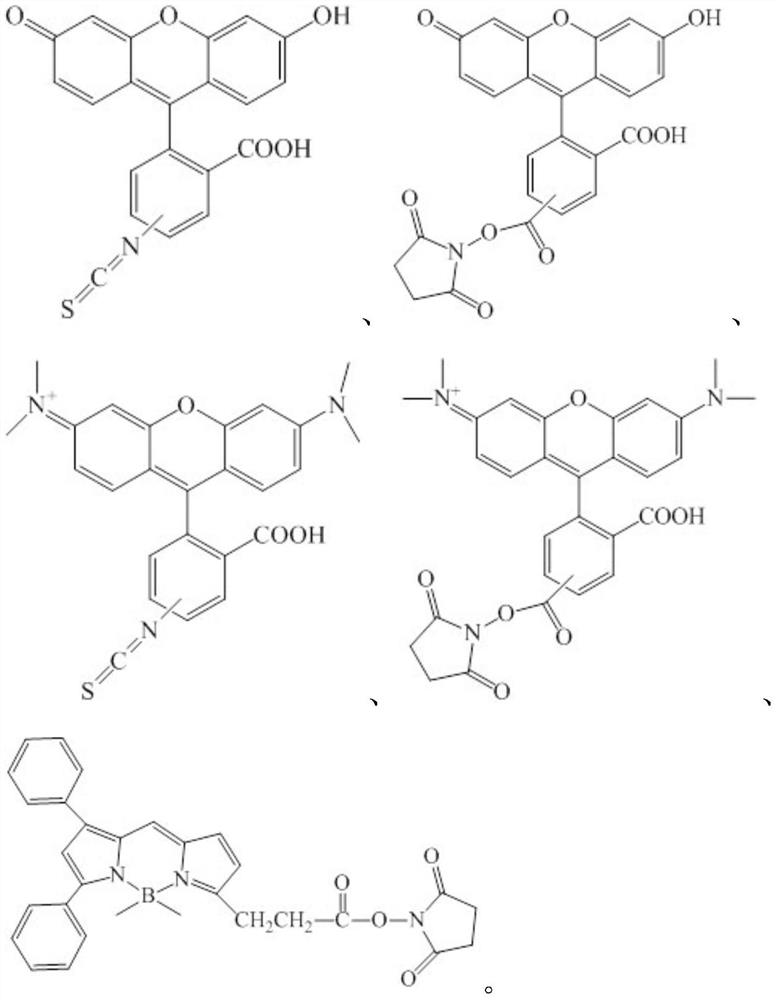
[0067] Preferably, the organic fluorescent molecules reactive with amino groups are selected from:
[0068] One of.
[0069] In the present invention, the reaction solvent I is N,N-dimethylfo...
preparation example 1
[0104] (1) Dissolve 1g of 4-fluoro-3-(trifluoromethyl)benzonitrile and 0.8g of 4-hydroxyl-3-methoxybenzaldehyde in 10mL of dimethylformamide (DMF), then add 2.2 K of g 2 CO 3 , heated to 65°C overnight to obtain the reaction solution I, the reaction solution I was diluted with 1mL of ice water, then extracted three times with 10mL of ethyl acetate, after the three extracts were combined, concentrated by drying, and passed through silica gel column chromatography (elution Liquid is acetic acid / normal hexane, volume ratio is 1:10) obtains the compound shown in the formula (III) of 1.4g pale yellow;
[0105] (2) 1.3g of the compound represented by formula (III), 515mg of 2,4-thiazolidinedione and 3.3g of sodium acetate (NaOAc) were added to 20mL of acetonitrile (ACN), at a temperature of 100°C After reacting for 24 hours to obtain reaction solution II, the organic phase of reaction solution II was spin-dried, and then purified by silica gel column chromatography (eluent was ace...
preparation example 2
[0109] (1) Dissolve 0.8g of 4-fluoro-3-(trifluoromethyl)benzonitrile and 0.7g of 4-hydroxyl-3-methoxybenzaldehyde in 10mL of dimethylformamide, then add 1.8g of Na 2 CO 3 , heated to 60°C overnight to obtain the reaction solution I, the reaction solution I was diluted with 1mL of ice water, then extracted three times with 10mL of ethyl acetate, after the three extracts were combined, dried and concentrated, passed through silica gel column chromatography (eluted Liquid is acetic acid / normal hexane, volume ratio is 1:10) obtains the compound shown in the pale yellow formula (III);
[0110] (2) Add 1g of the compound represented by formula (III), 0.4g of 2,4-thiazolidinedione and 2.4g of potassium acetate into 20mL of acetonitrile, and react for 30h at a temperature of 80°C to obtain a reaction solution II, the organic phase of the reaction solution II is spin-dried, and then purified by silica gel column chromatography (the eluent is acetic acid / n-hexane, the volume ratio is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com