Interferon composition as well as preparation method and application thereof
A composition and technology of interferon, applied in the field of biomedicine, can solve problems such as high difficulty in preservation, high cost of human serum albumin, strong immunogenicity, etc., to avoid intolerance, high difficulty in preservation, and high difficulty in preparation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
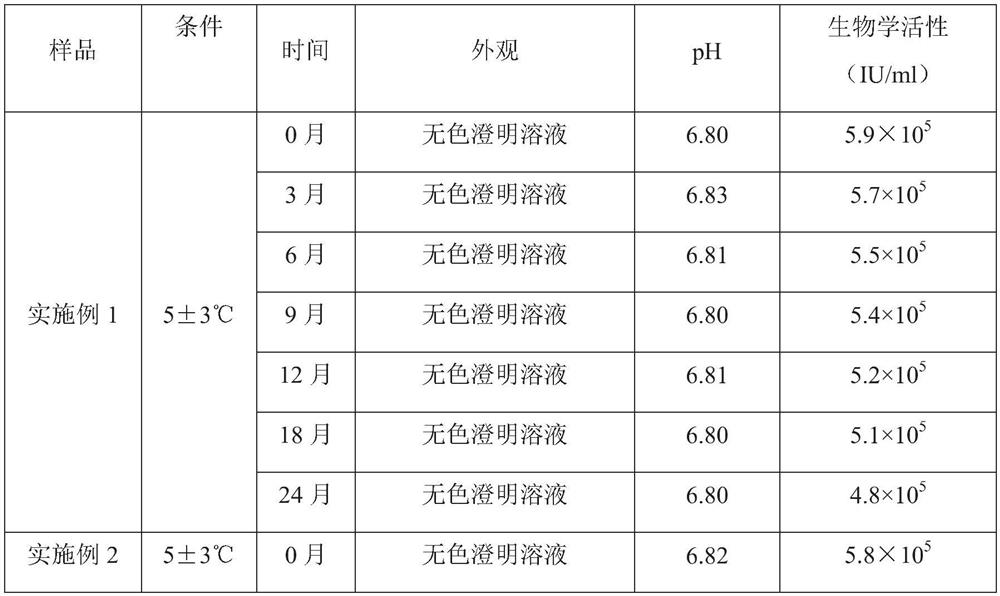
[0060] Embodiment 1: the preparation of interferon composition
[0061] Prescription: as shown in Table 1.
[0062] Table 1: Prescriptions for Interferon Compositions
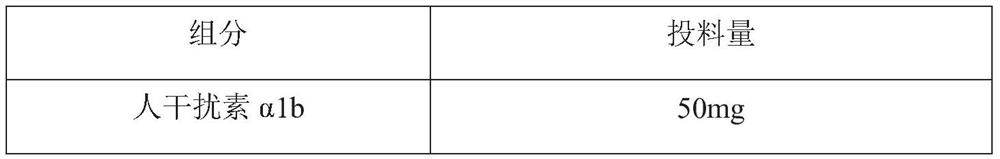
[0063] components Feeding amount human interferon alpha 1b 50mg hyaluronic acid 2.0g Disodium hydrogen phosphate dodecahydrate 0.3g Sodium dihydrogen phosphate monohydrate 0.1g Sodium chloride 7.0g Tween 80 0.1g Edetate Disodium 0.2g Water for Injection Add water for injection to make the composition volume 1000mL
[0064] Preparation process: Take hyaluronic acid, add water for injection, heat and stir until completely dissolved, cool to room temperature, add disodium hydrogen phosphate dodecahydrate, sodium dihydrogen phosphate monohydrate, sodium chloride, Tween 80 and disodium edetate , stirred to dissolve, then added human interferon α1b, stirred evenly, sterilized and filtered to obtain an interferon composition.
Embodiment 2
[0065] Embodiment 2: the preparation of interferon composition
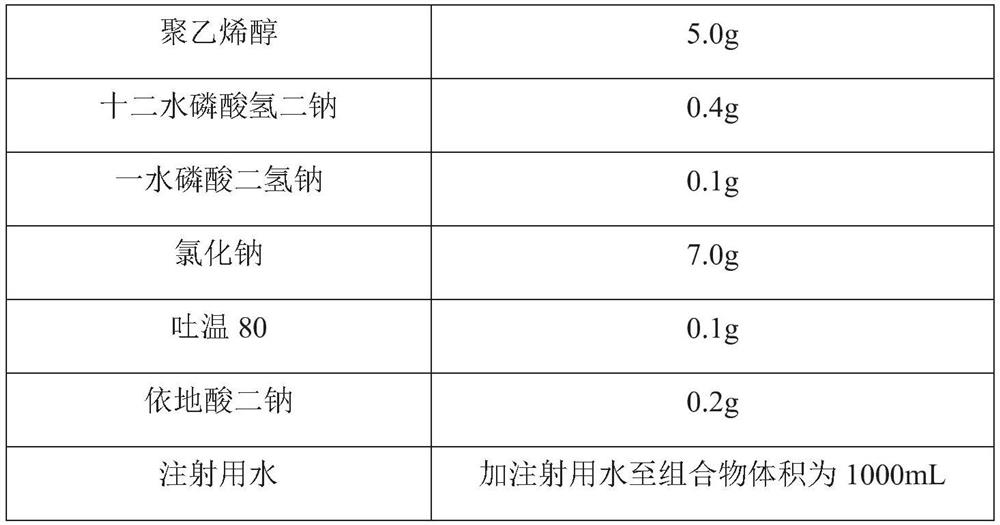
[0066] Prescription: as shown in Table 2.
[0067] Table 2: Prescriptions for Interferon Compositions
[0068]
[0069]
[0070] Preparation process: Take polyvinyl alcohol, add water for injection, heat and stir until completely dissolved, cool to room temperature, add disodium hydrogen phosphate dodecahydrate, sodium dihydrogen phosphate monohydrate, sodium chloride, Tween 80 and disodium edetate , stirred to dissolve, then added human interferon α1b, stirred evenly, sterilized and filtered to obtain an interferon composition.
Embodiment 3
[0071] Embodiment 3: the preparation of interferon composition
[0072] Prescription: as shown in Table 3.
[0073] Table 3: Prescriptions for Interferon Compositions
[0074] components Feeding amount human interferon alpha 1b 50mg hyaluronic acid 2.0g Trehalose 10.0g Disodium hydrogen phosphate dodecahydrate 0.4g Sodium dihydrogen phosphate monohydrate 0.1g Sodium chloride 7.0g Tween 80 0.1g Edetate Disodium 0.2g Water for Injection Add water for injection to make the composition volume 1000mL
[0075] Preparation process: Take hyaluronic acid, add water for injection, heat and stir until completely dissolved, cool to room temperature, add trehalose, disodium hydrogen phosphate dodecahydrate, sodium dihydrogen phosphate monohydrate, sodium chloride, Tween 80 and editidine disodium acid, stirred and dissolved, then added human interferon α1b, stirred evenly, sterilized and filtered to obtain the interfero...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com