Preparation method of chlorinated linezolid impurity
A technology of linezolid and chlorination, which is applied in the field of medicine, can solve the problems of inability to obtain pure impurities and low conversion rate, and achieve the effect of reducing the risk of use, high conversion rate and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
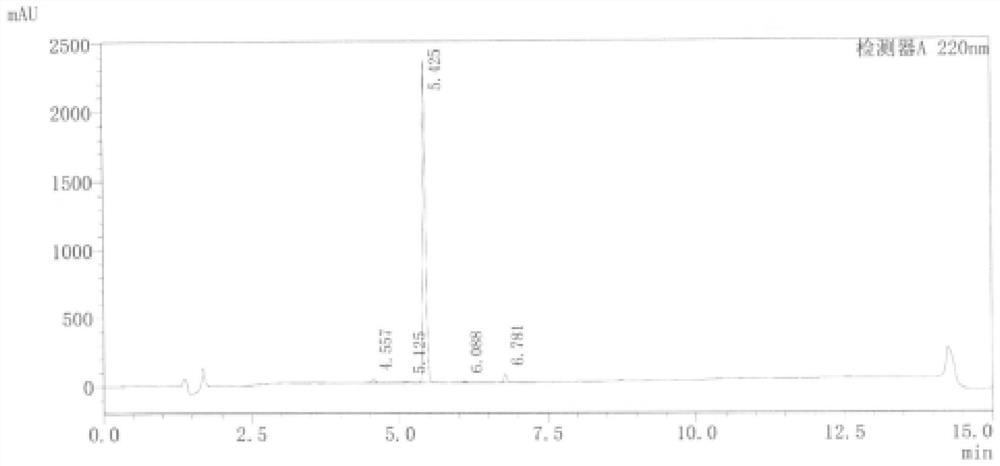
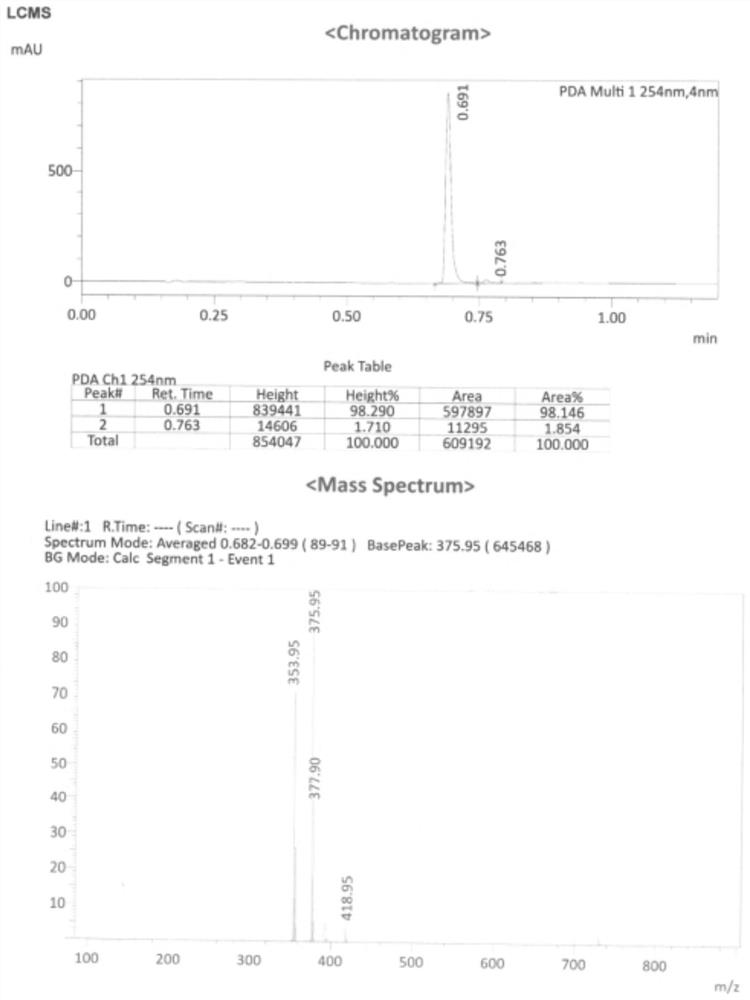
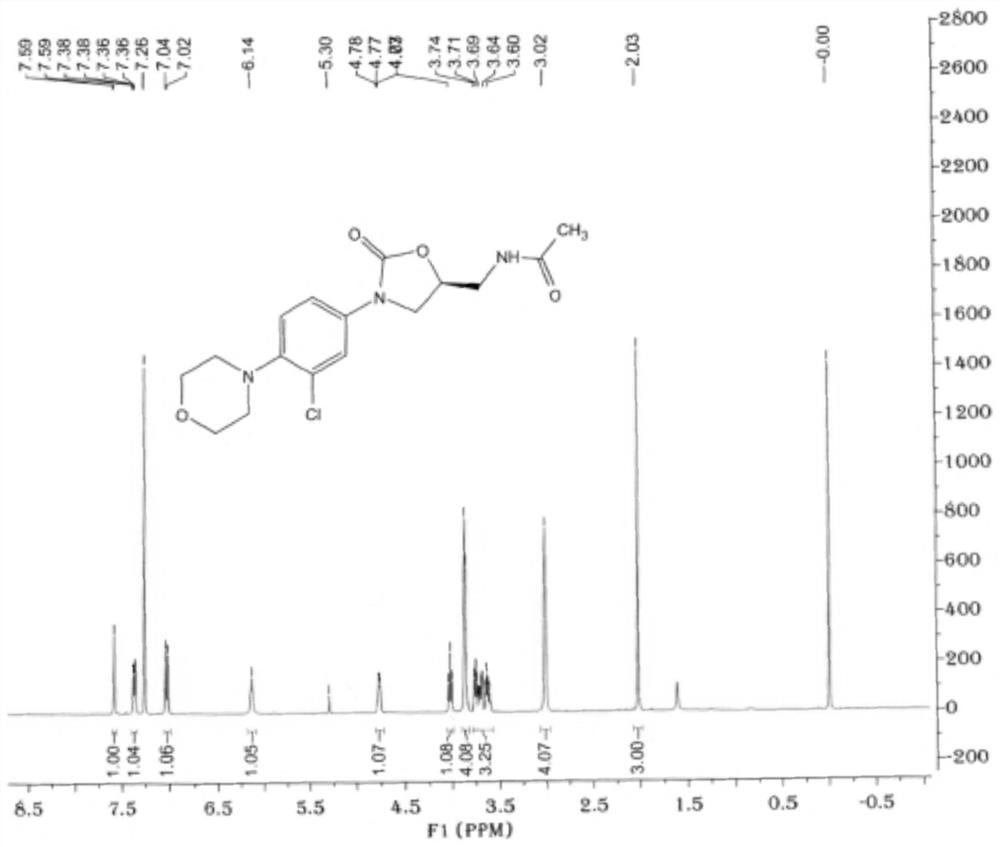
Embodiment 1
[0048] This embodiment provides a linezolid chlorinated impurity represented by formula V, and its reaction equation and preparation method are as follows:
[0049]
[0050] (1) Take 3.0g of the compound of formula I, 2.0g of potassium phthalimide, and 30ml of DMF and heat together for 5 hours at a temperature of 115±5°C. After the reaction is completed, add water to precipitate the product, filter and dry, 3.5 g of N-(3-phthalimido-2-hydroxypropyl)-3-chloro-4-morpholinoaniline (compound of formula II) was obtained;
[0051] (2) Preparation of N-[3-[(3-chloro-4-morpholinophenyl)-2-oxo-5-oxazolidinyl]methyl]-phthalimide: Mix 3.5 g of the compound of formula II obtained in the previous step, 2.0 g of carbonyldiimidazole (CDI), and 40 g of dichloromethane, react at room temperature for 20 hours, wash with water after the reaction, dry over anhydrous sodium sulfate, and evaporate to dryness under reduced pressure. Then recrystallize with ethanol to get N-[3-[(3-chloro-4-morpho...
Embodiment 2
[0059] This embodiment provides a linezolid chlorinated impurity represented by formula V, and its reaction equation and preparation method are as follows:
[0060]
[0061] (1) Heat 32.1g of the compound of formula I, 20.0g of potassium phthalimide, and 320ml of DMF at a temperature of 115±5°C for 5 hours. After the reaction, add water to precipitate the product, filter and dry, Obtained 32.8g, and recrystallized with isopropanol to obtain 16.1g of N-(3-phthalimido-2-hydroxypropyl)-3-chloro-4-morpholinoaniline (compound of formula II);
[0062] (2) 16.0 g of the compound of formula II obtained in the previous step, 8.0 g of triphosgene, 10.0 g of triethylamine, and 100 ml of dichloromethane were mixed, reacted at room temperature for 20 hours, washed with water after the reaction, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure . Then recrystallize with ethanol to get N-[3-[(3-chloro-4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]methyl]-p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com