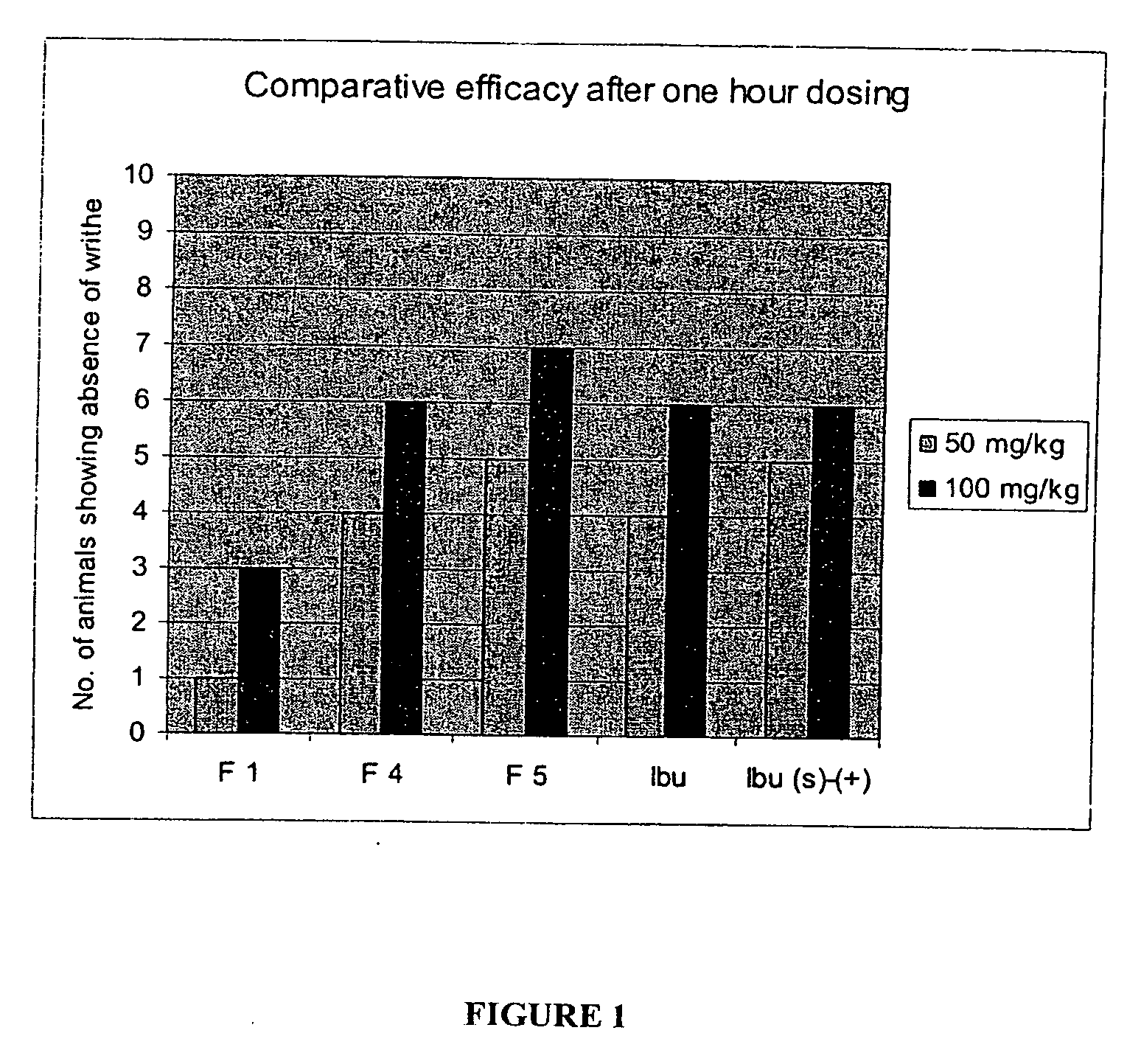
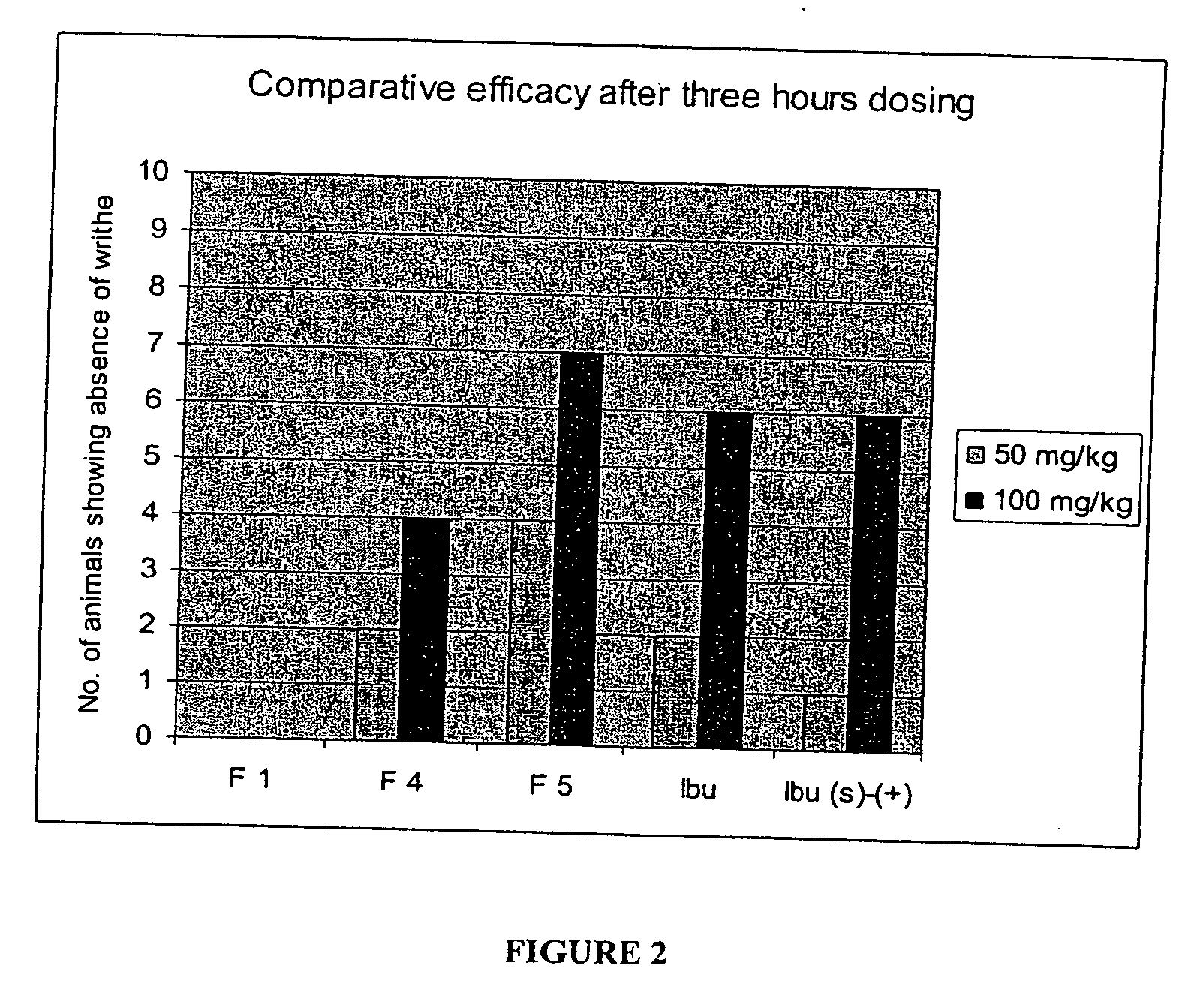
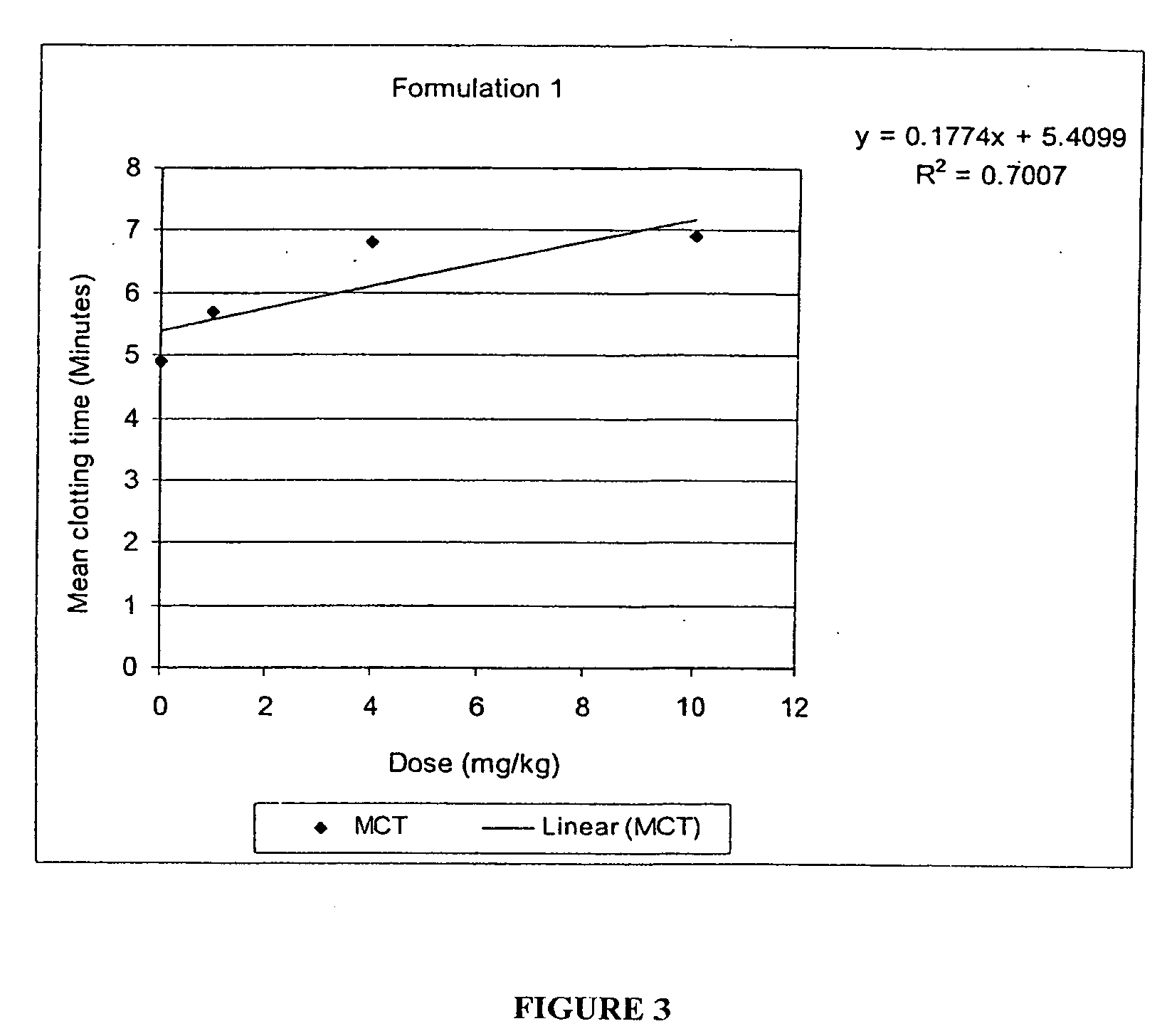
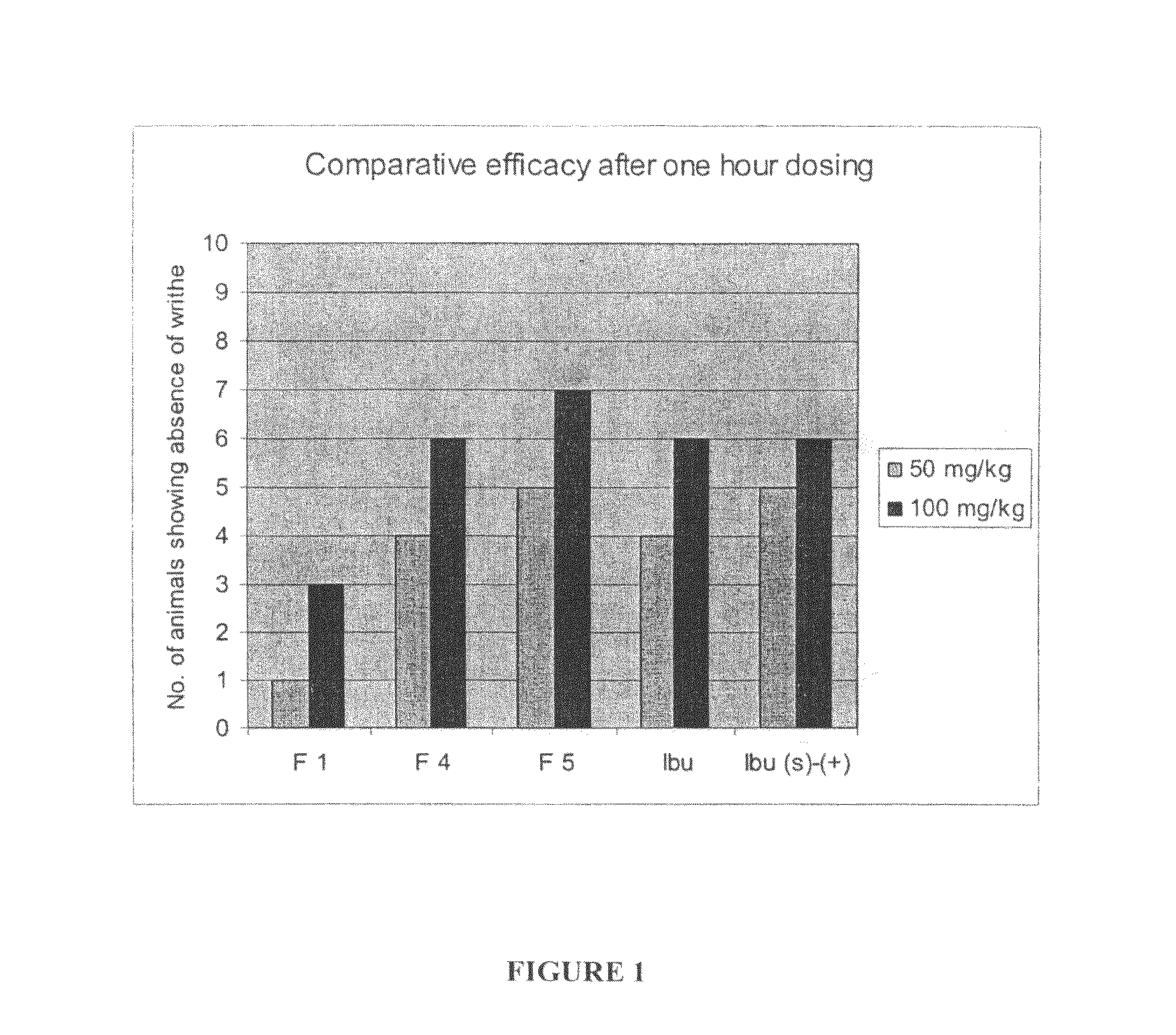
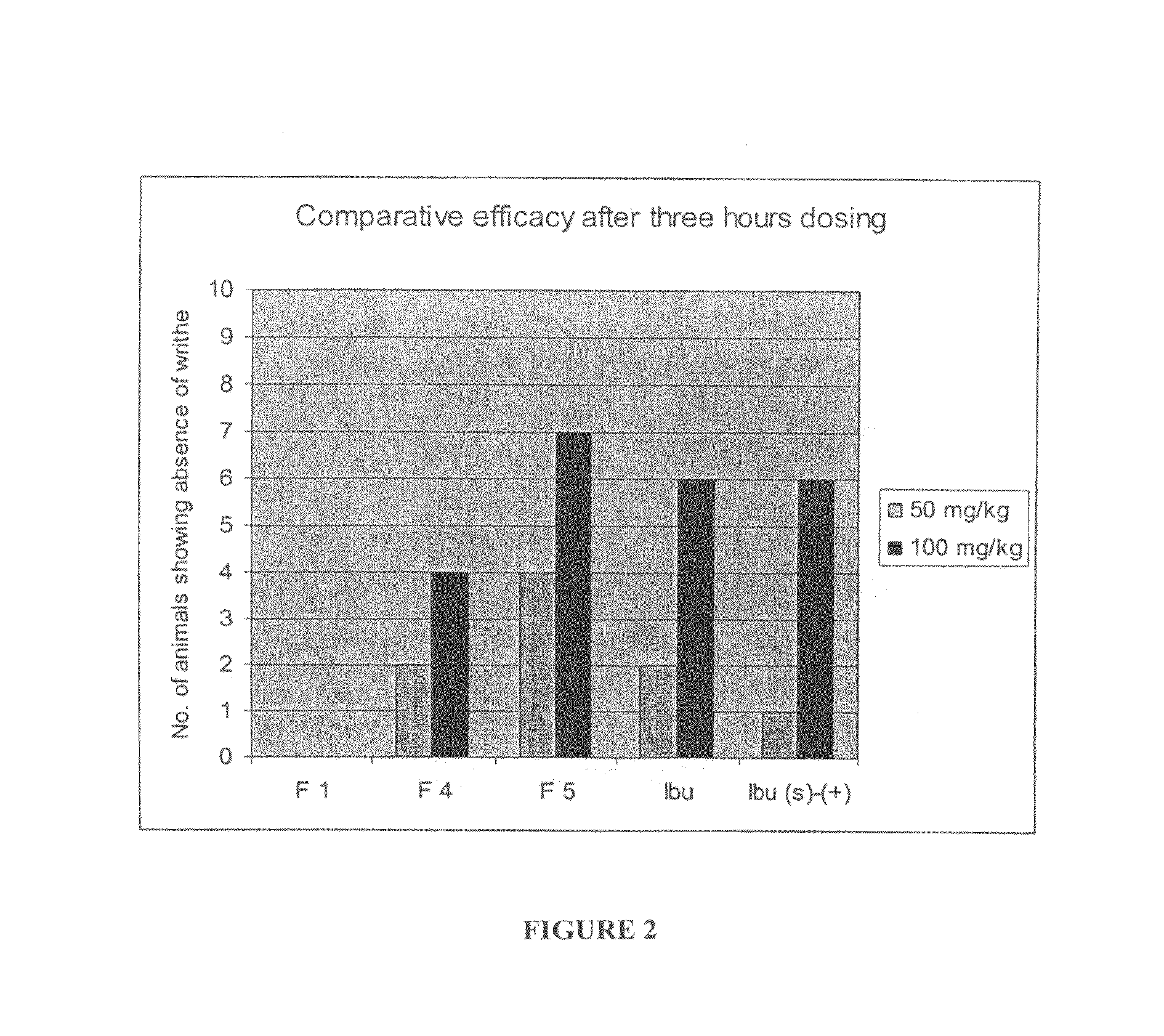
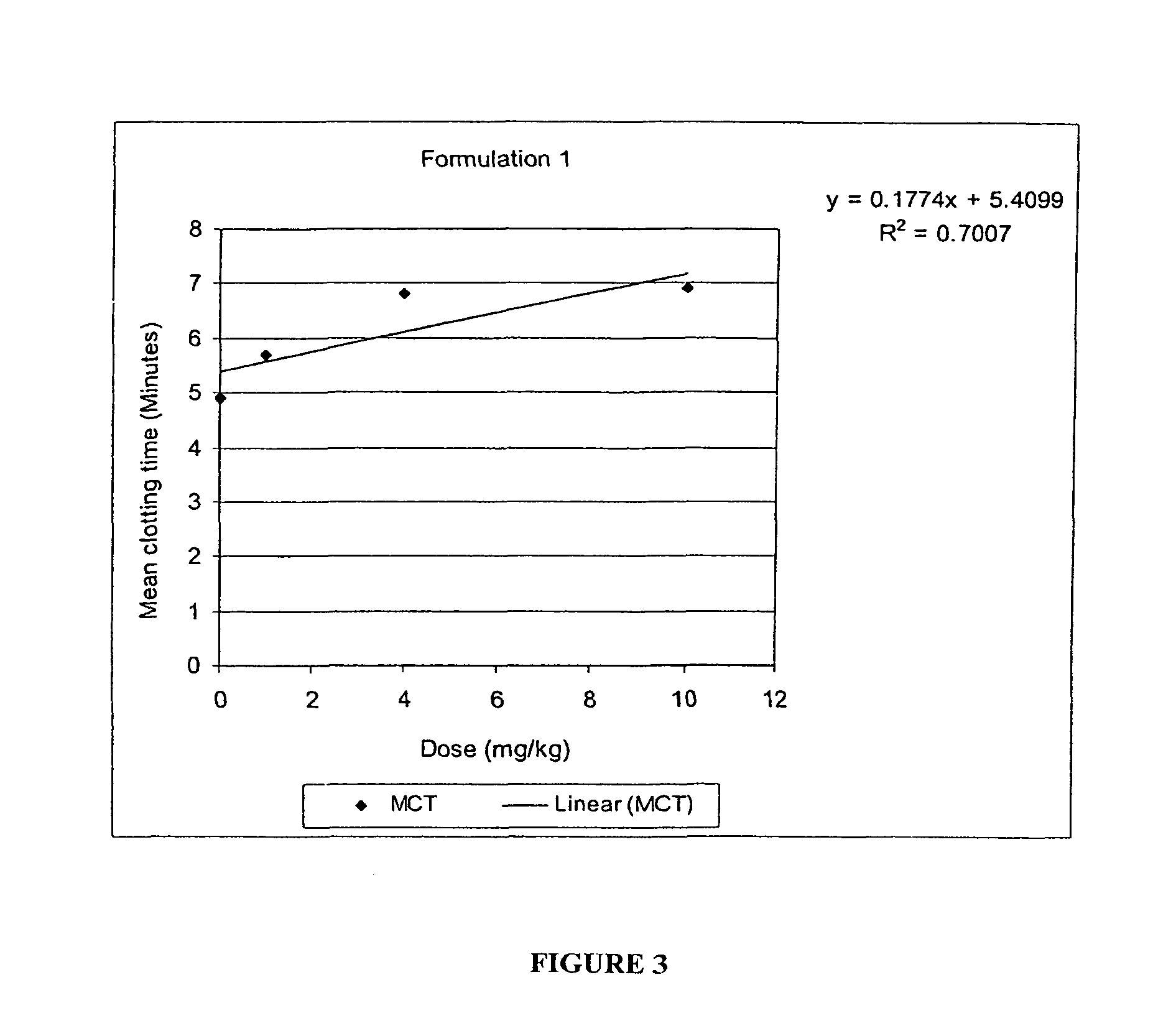
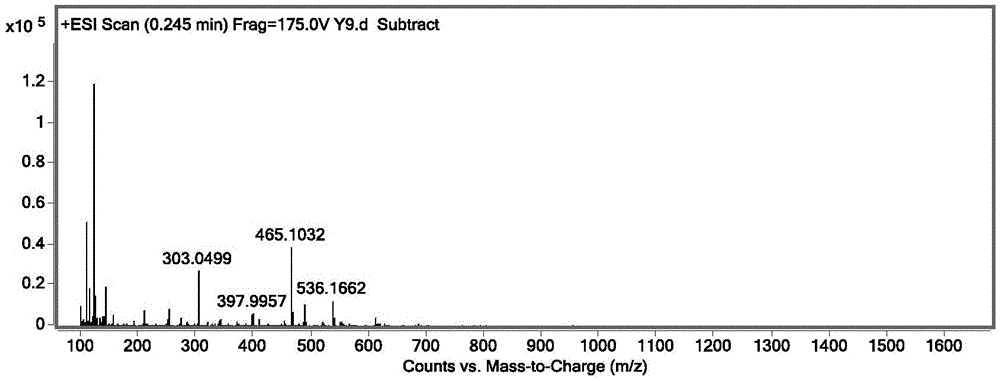
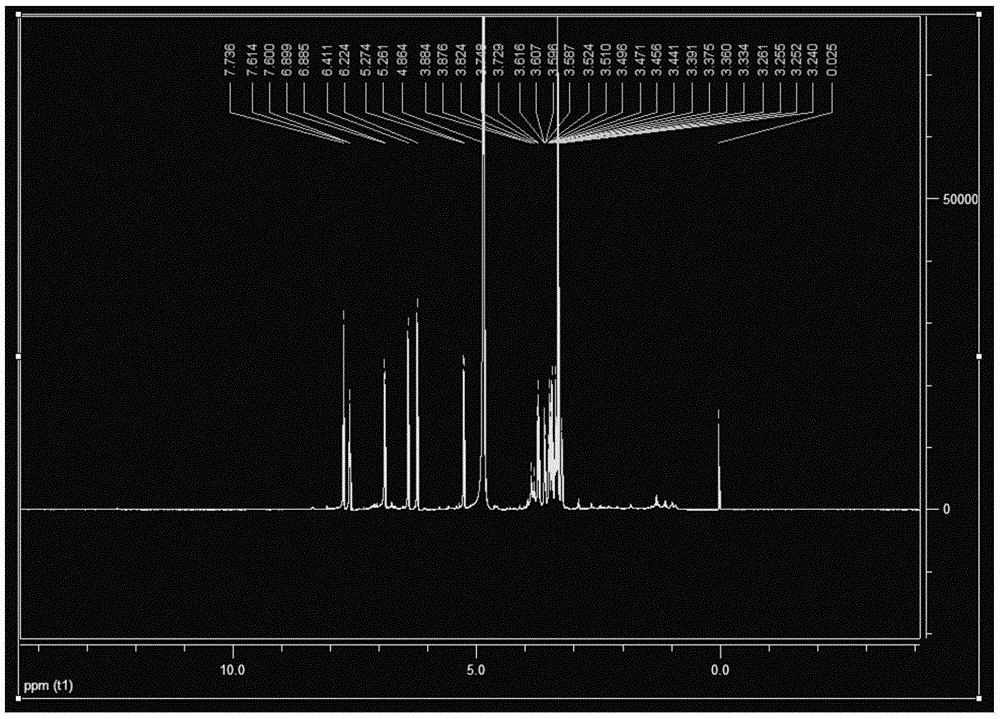
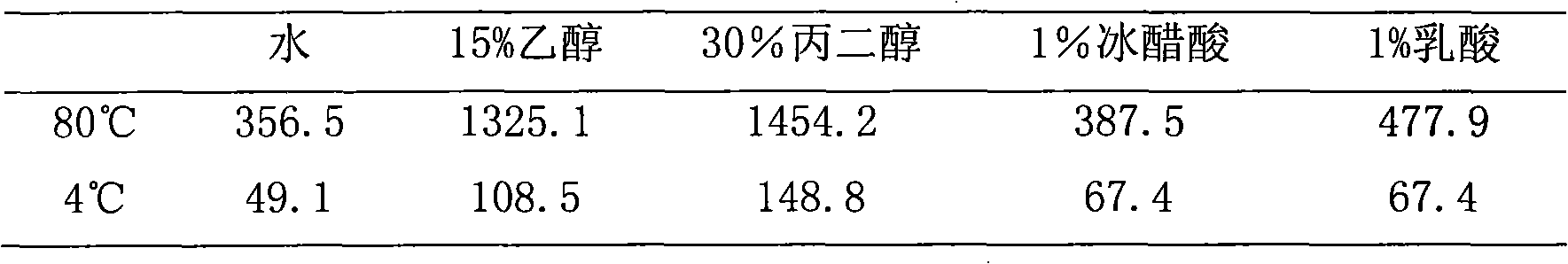
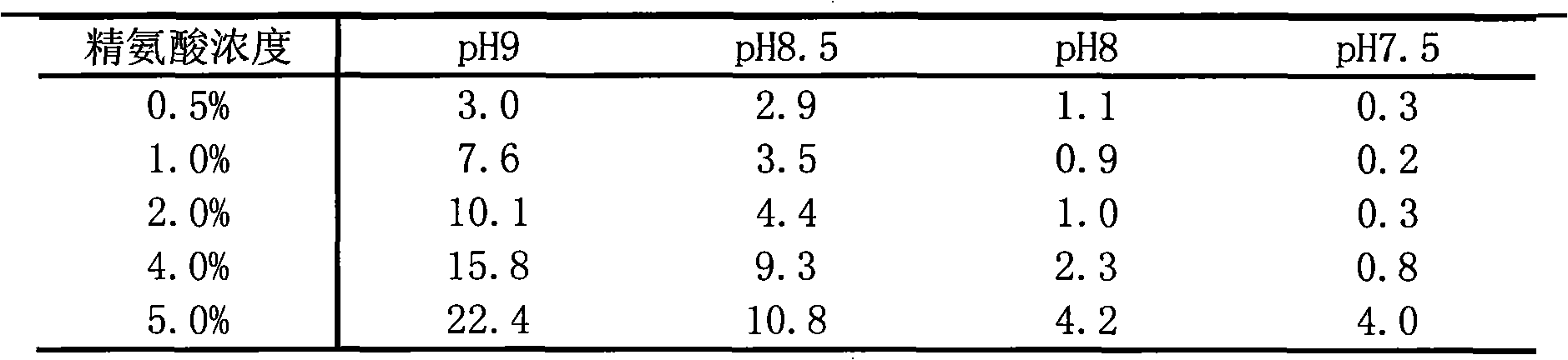
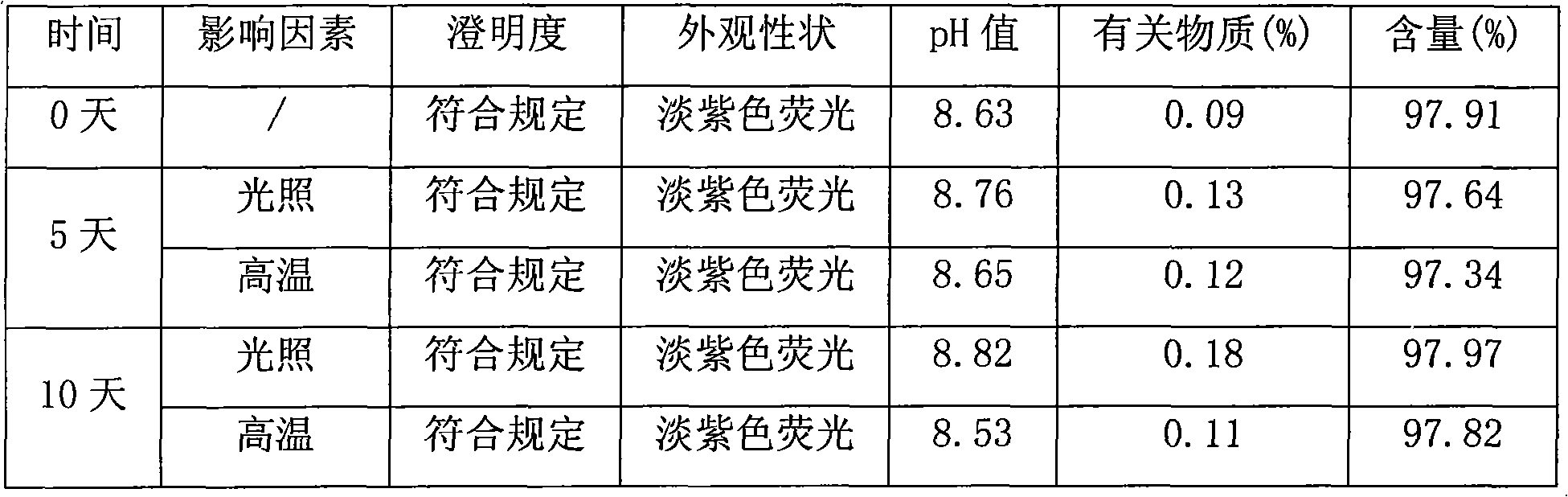
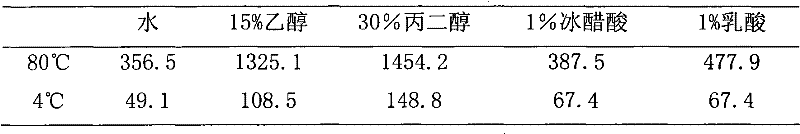
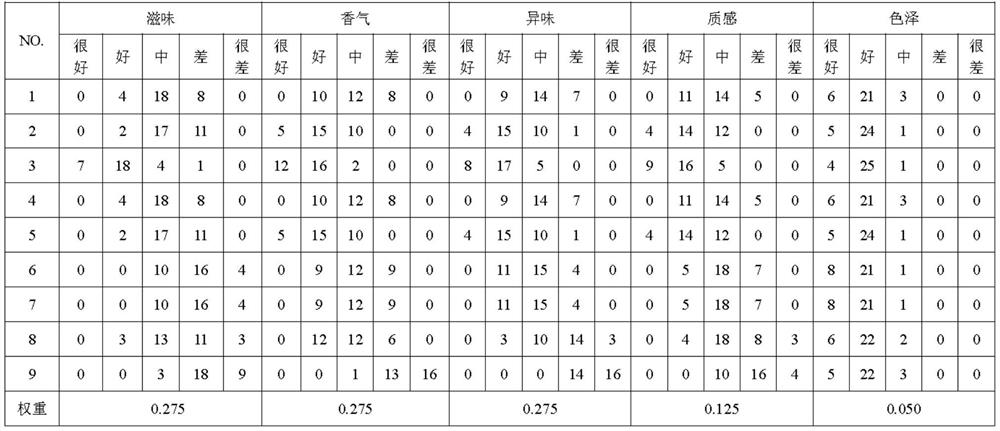
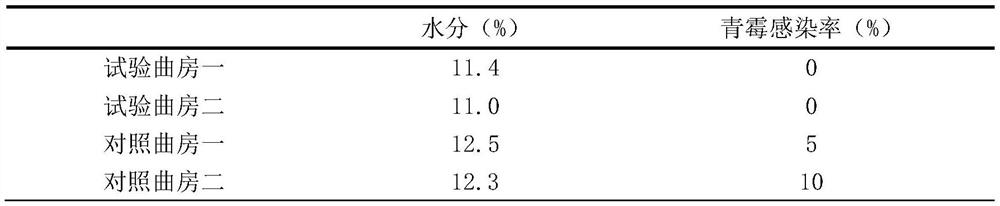
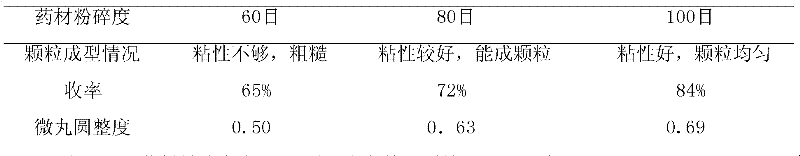
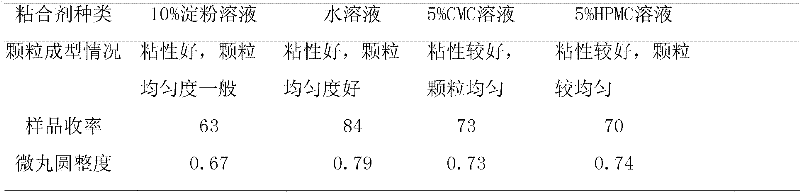
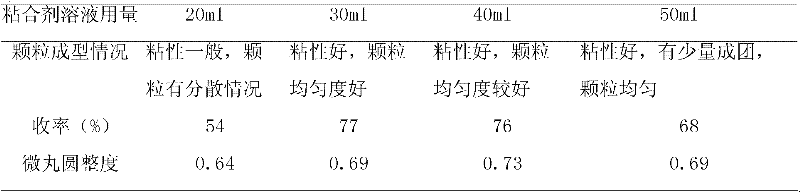
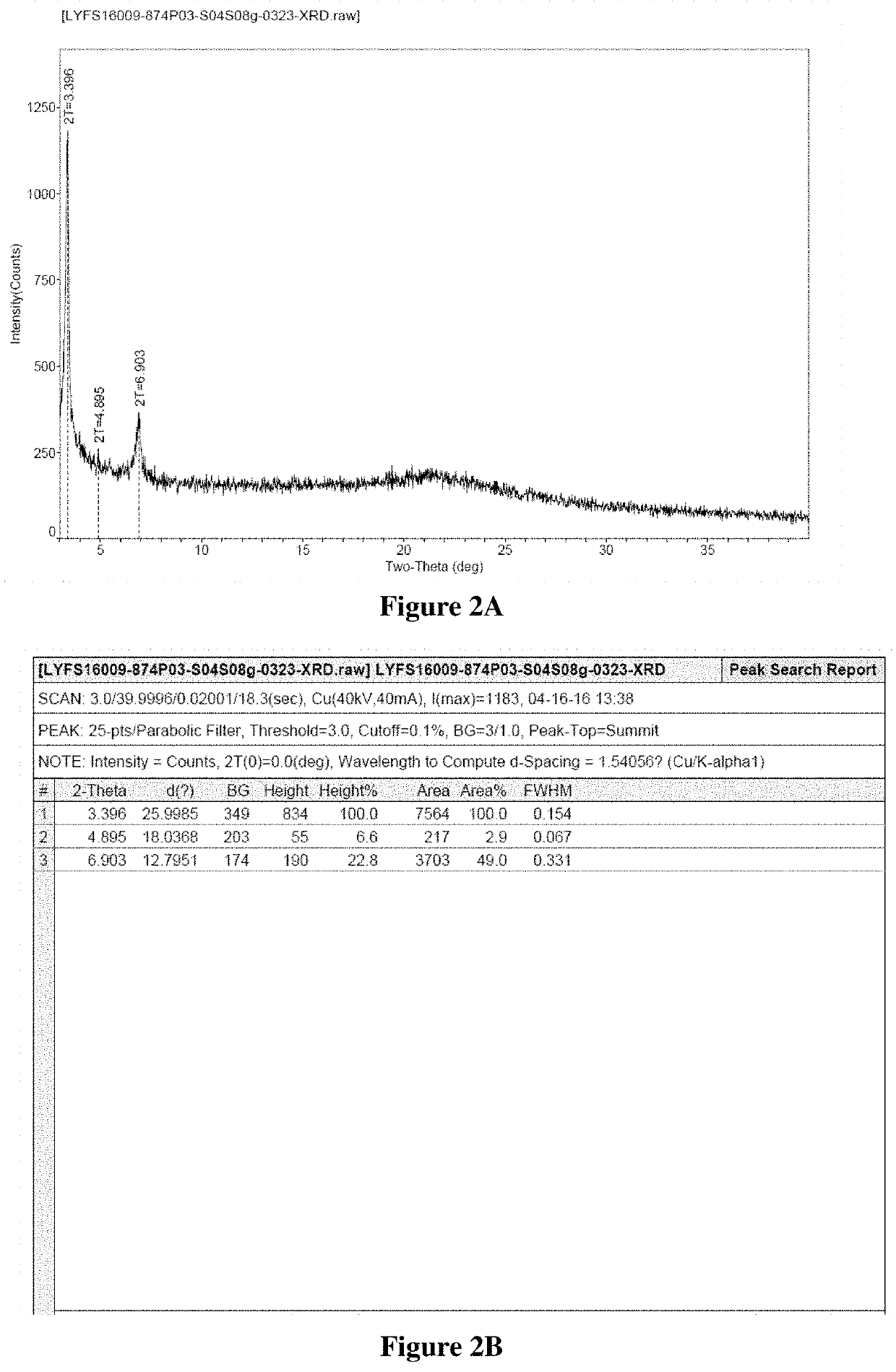
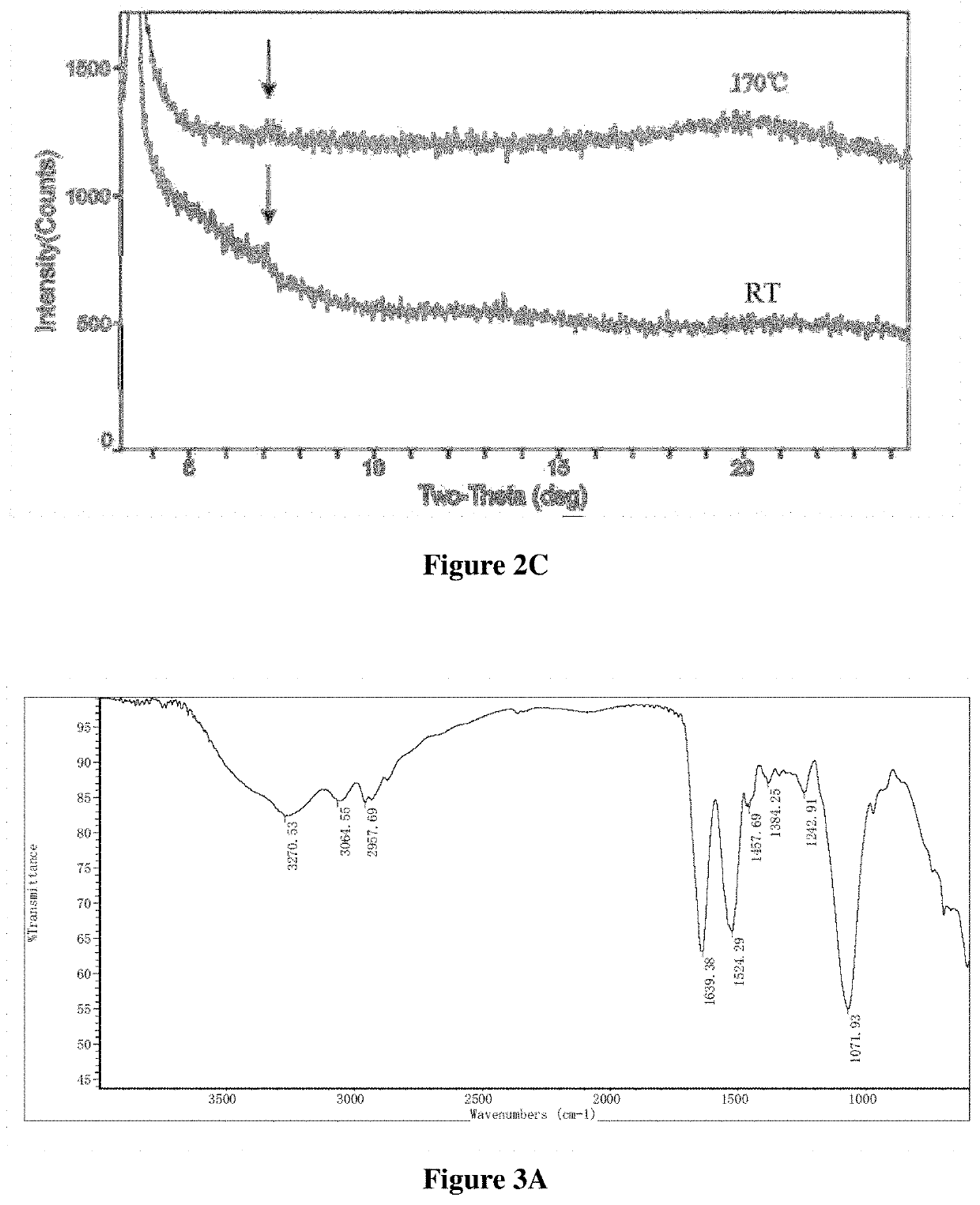
Patents
Literature
34results about How to "Improve drug quality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel compounds with high therapeutic index
ActiveUS20060241017A1Improve bioavailability and efficacyLess toxicBiocideAntipyreticAmino acid compositionCompound (substance)
The present invention is directed to novel therapeutic compounds comprised of an amino acid bonded to a medicament or drug having a hydroxy, amino, carboxy or acylating derivative thereon. These high therapeutic index derivatives have the same utility as the drug from which they are made, and they have enhanced pharmacological and pharmaceutical properties. In fact, the novel drug derivatives of the present invention enhance at least one therapeutic quality, as defined herein. The present invention is also directed to pharmaceutical compositions containing same.
Owner:SIGNATURE R & D HLDG LLC
Compounds with high therapeutic index
The present invention is directed to novel therapeutic compounds comprised of an amino acid bonded to a medicament or drug having a hydroxy, amino, carboxy or acylating derivative thereon. These high therapeutic index derivatives have the same utility as the drug from which they are made, and they have enhanced pharmacological and pharmaceutical properties. In fact, the novel drug derivatives of the present invention enhance at least one therapeutic quality, as defined herein. The present invention is also directed to pharmaceutical compositions containing same.
Owner:SIGNATURE R & D HLDG LLC
Anti-tumor traditional Chinese medicine and preparation method thereof
InactiveCN106075141AGood effectInhibit and reduce tumorAmphibian material medical ingredientsOrganic active ingredientsTreatment effectPericarpium citri reticulatae
The invention discloses an anti-tumor traditional Chinese medicine. 60-80 parts of sculellaria barbata, 70-90 parts of radix bupleuri, 70-90 parts of cordyceps sinensis, 50-70 parts of American ginseng, 40-60 parts of cyrtomium rhizomes, 30-60 parts of pericarpium citri reticulatae viride and 40-60 parts of isatis roots are added in the formula of the anti-tumor traditional Chinese medicine, a proper number of auxiliary materials are added, 1,000 tablets are prepared, coating is carried out, and the anti-tumor traditional Chinese medicine is prepared. A preparation process of the anti-tumor traditional Chinese medicine is improved. On the premise of guaranteeing that the medicine treatment effect is basically stable, stability of the anti-tumor traditional Chinese medicine in the production process, the medicine quality and the medicine effect are improved, the useful life of the anti-tumor traditional Chinese medicine is prolonged, all ingredient content is still larger than 99.6% after the anti-tumor traditional Chinese medicine is placed for 36 month at room temperature, and the production process of the anti-tumor traditional Chinese medicine achieves substantive breakthrough and technical progress.
Owner:GUIZHOU MEDICAL UNIV
Traditional Chinese medicine composition formed by ginkgo biloba extract and application thereof to preparation of Shuxuening parenteral solution
InactiveCN105641004AImprove efficacyLess impuritiesOrganic active ingredientsSugar derivativesDrugQuercitrin
The invention discloses a traditional Chinese medicine composition formed by ginkgo biloba extract. The traditional Chinese medicine composition is prepared from, by mass, 24-40% of gingko total flavone, 6-16% of bilobalide, and ginkgolic acid with the amount smaller than 5 ppm. Preferably, the traditional Chinese medicine composition is further prepared from 1-1.68% of quercetin-3-O-glucoside and 2.11-3.53% of quercetin-3-O-2'',6''-dirhamnose glucoside. Preferably, the traditional Chinese medicine composition is further prepared from 1.4-3% of bilobalide A, 0.9-1.8% of bilobalide B and 1.2-1.3% of bilobalide C. Quercetin-3-O-glucoside and quercetin-3-O-2'',6''-dirhamnose glucoside having an important influence on the medicine are definite, the content of quercetin-3-O-glucoside and the content of quercetin-3-O-2'',6''-dirhamnose glucoside are accurately defined as 1-1.68% and 2.11-3.53%, furthermore, biobalide A is clearly defined as 1.4-3%, biobalide B is clearly defined as 0.9-1.8% and biobalide B is clearly defined as 1.2-1.3%. By making the content of the effective components in the ginkgo biloba extract to be accurate and further affirming the effective components, efficacy of the ginkgo biloba extract is improved.
Owner:北京华润高科天然药物有限公司
Compound preparations for treating hypertension and method for preparing the same
InactiveCN101134032AReliable and safe pressure reducing effectReasonable collocationPill deliveryCardiovascular disorderCompounded preparationsTreatment hypertension
The present invention discloses one kind of hypertension treating compound preparation and its preparation process. The compound preparation includes one tablet core containing diuretic in effective treating amount and pharmaceutically acceptable carrier, and one coating containing telmisartan in effective treating amount and pharmaceutically acceptable carrier. The content of diuretic is 1.5-10 wt% of the tablet core, and that of telmisartan is 3.5-25 wt% of the coating. The compound preparation is prepared through preparing the tablet core pellet and the coating pellet separately with medicine material and supplementary material and the subsequent pressing the coated tablet in a rotary coating machine. The hypertension treating compound preparation has reliable and safe blood pressure reducing effect and high stability.
Owner:湖北丝宝药业有限公司
Method for controlling antiviral oral liquid
A quaility control method for the antiviral oral liquid features that on the basis of existing quality control standards, the gas-phase chromatography method is additionally used to measure the content for patchoulic alcohol to ensure that it is not less than 10 micrograms per 10 ml.
Owner:GUANGZHOU XIANGXUE PHARMA CO LTD
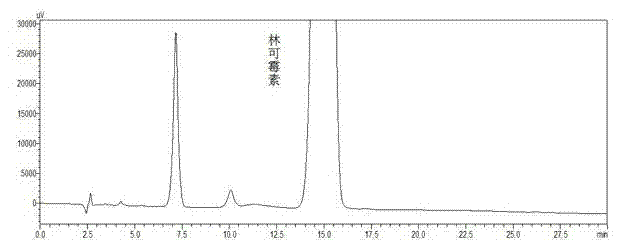
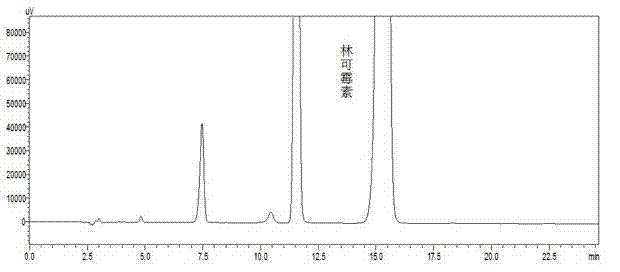
Preparation method and applications of dehydro-lincomycin-free lincomycin hydrochloride
ActiveCN102964400AReduce drug impuritiesImprove drug qualityAntibacterial agentsOrganic active ingredientsLincomycin HydrochloridePalladium on carbon
The invention relates to a preparation method and applications of dehydro-lincomycin-free lincomycin hydrochloride, which belong to the field of medical technologies. The preparation method disclosed by the invention comprises the following steps: dissolving dehydro-lincomycin-containing lincomycin hydrochloride serving as a raw material into water or an aqueous alcohol solution; adding palladium on carbon accounting for 1 / 10-1 / 200 of the amount of the raw material, and simultaneously stirring and introducing hydrogen into the obtained mixture to react for 1-48 hours, so that dehydro-lincomycin is all reacted to generate lincomycin; and crystallizing lincomycin. According to the preparation method, dehydro-lincomycin in lincomycin hydrochloride has a chemical reaction to generate lincomycin hydrochloride, so that dehydro-lincomycin in the raw material is completely removed; and the raw material can be used for preparing a dehydro-lincomycin-free lincomycin hydrochloride preparation, reducing the impurities in pharmaceutical products, and improving the quality of pharmaceutical products.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Capsule real-time inspection method with double detection
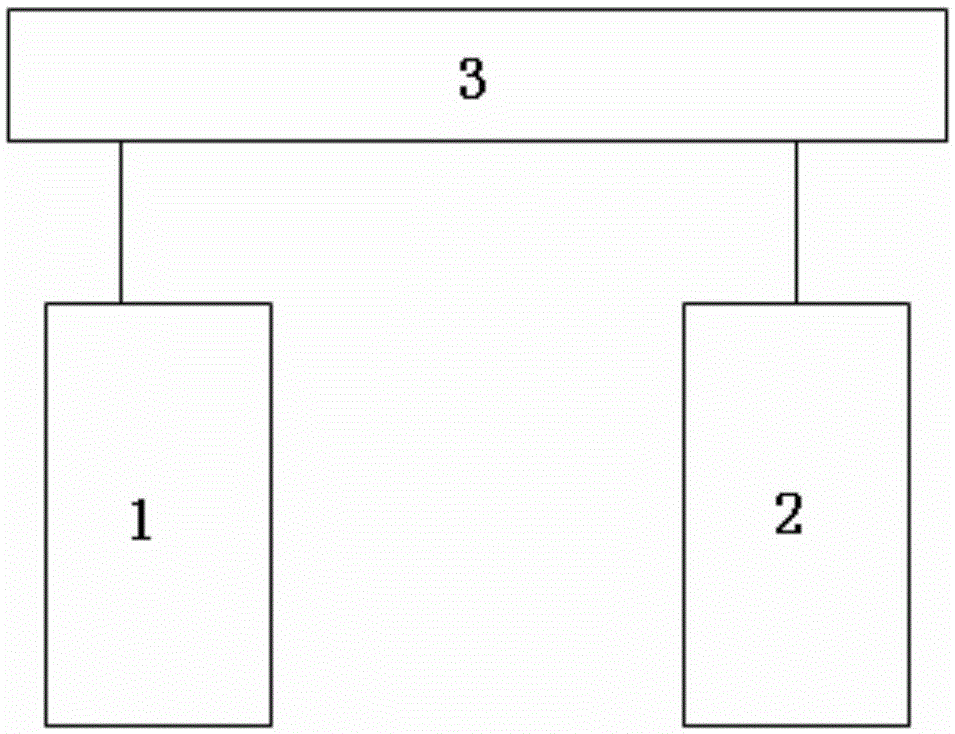
ActiveCN105241890AImprove drug qualityRemoval in timeOptically investigating flaws/contaminationSortingEngineeringMaster controller
The invention relates to a capsule real-time inspection method with double detection, which comprises the following steps: 1) providing a capsule real-time test platform with double detection, which includes a near-infrared detection mechanism, a visible light detection mechanism and a main controller, wherein the near-infrared detection mechanism and the visible light detection mechanism respectively perform defect detection to every capsule and the main controller is respectively connected to the near-infrared detection mechanism and the visible light detection mechanism for determining a capsule rejection strategy according to a defect detection result from the near-infrared detection mechanism and the visible light detection mechanism; and 2) performing inspection with the test platform. According to the method, there are two detection methods are available to detect the capsules, thereby improving reliability of capsule test.
Owner:NANNING FRESH LIFE BIOTECH
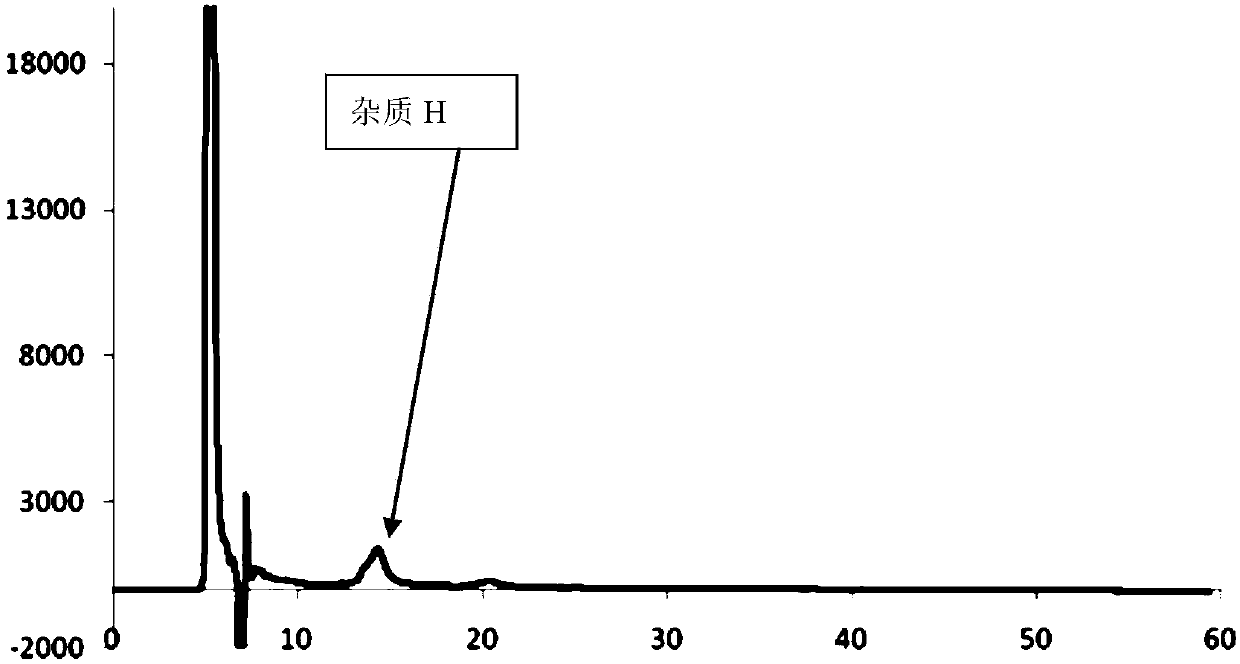
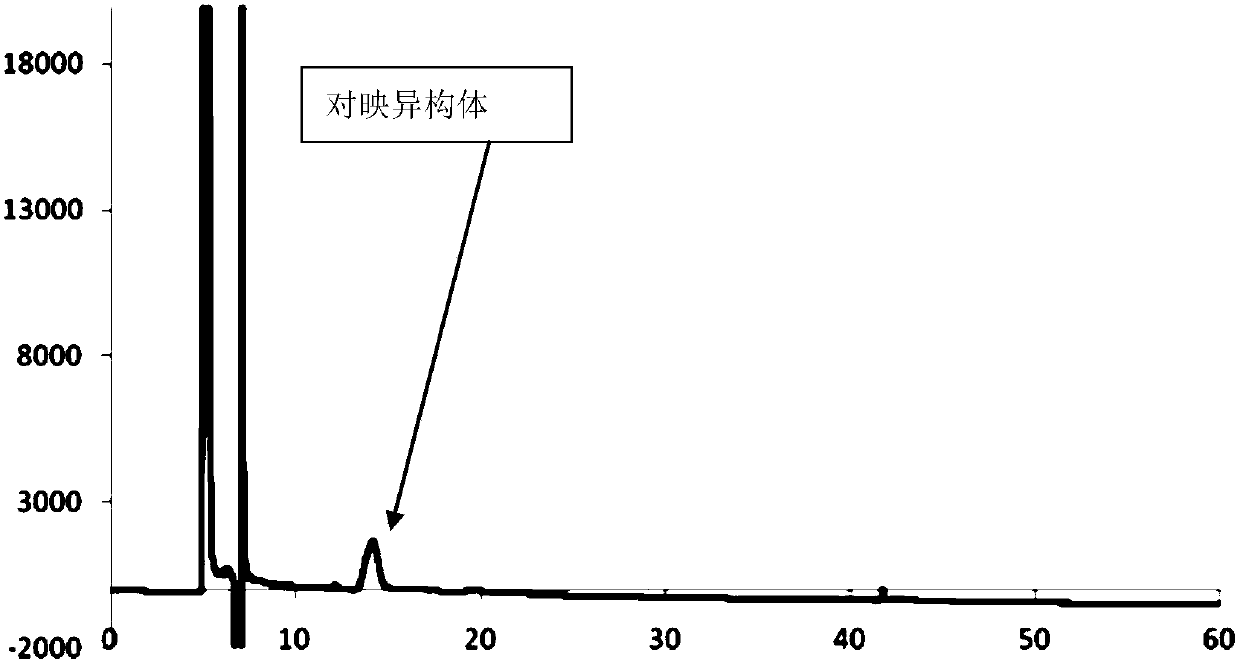
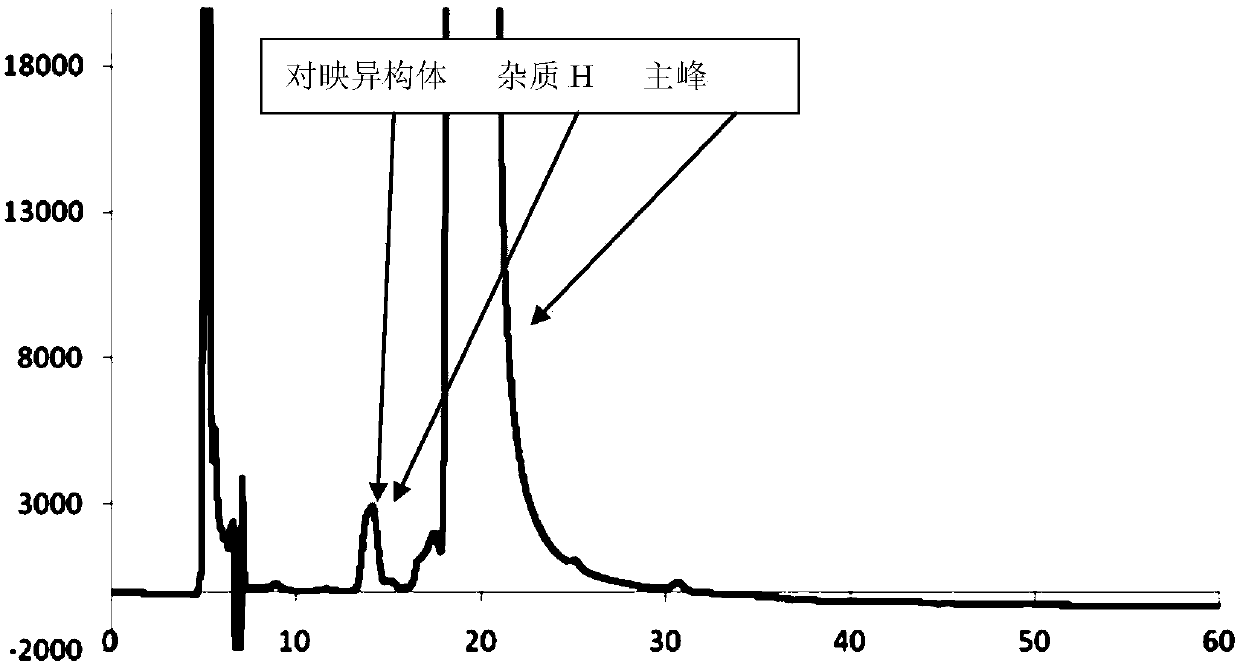
Separation and/or detection method of impurities in atorvastatin calcium
The invention relates to a separation and / or detection method of impurities in atorvastatin calcium. The impurities are atorvastatin calcium enantiomers and / or atorvastatin calcium lactone (impurity H). The method uses high-performance liquid chromatography for separation or detection. A mobile phase of the high-performance liquid chromatography is monohydric alcohol-organic acid-organic base of n-hexane-C1-C6. A volume ratio of the monohydric alcohol-organic acid-organic base of the n-hexane-C1-C6 is 85-98:2-15:0.05-0.5:0.05-0.5. The method can realize effective separation on the three partsof the atorvastatin calcium, the atorvastatin calcium enantiomers and the atorvastatin calcium lactone; and at the same time, the method of the invention can be used for detection of the atorvastatincalcium enantiomers and the atorvastatin calcium lactone, and can effectively improve medicine quality and safety of the atorvastatin calcium.
Owner:北京伟林恒昌医药科技有限公司
Highly-pure tacrolimus compound and preparation method thereof
ActiveCN106083892AImprove drug qualityHigh purityOrganic chemistryImmunological disordersDrugSolvent
The invention relates to the technical field of medicine purification, and discloses a highly-pure tacrolimus compound and a preparation method thereof. The method comprises the following steps: preprocessed crude tacrolimus undergoes silver-containing polymer resin column chromatography elution, the above obtained eluate is concentrated, and the above obtained concentrate is re-crystallized to obtain highly-pure tacrolimus. The method has the advantages of simplicity in large-scale production operation, small use amount of a solvent, short production period, low cost and environmental protection; and an HPLC detection result shows that ascomycin and dihydrotacrolimus which are most difficult to separate in the crude tacrolimus are completely removed. The normalization purity of tacrolimus is 99% or above, and obtained tacrolimus crystals have the characteristics of uniform particle size distribution, small internal stress and bulk density, and good stability, fluidity, dispersibility and dissolvability. The quality and the medication safety of tacrolimus are improved.
Owner:HANGZHOU ZHONGMEI HUADONG PHARMA
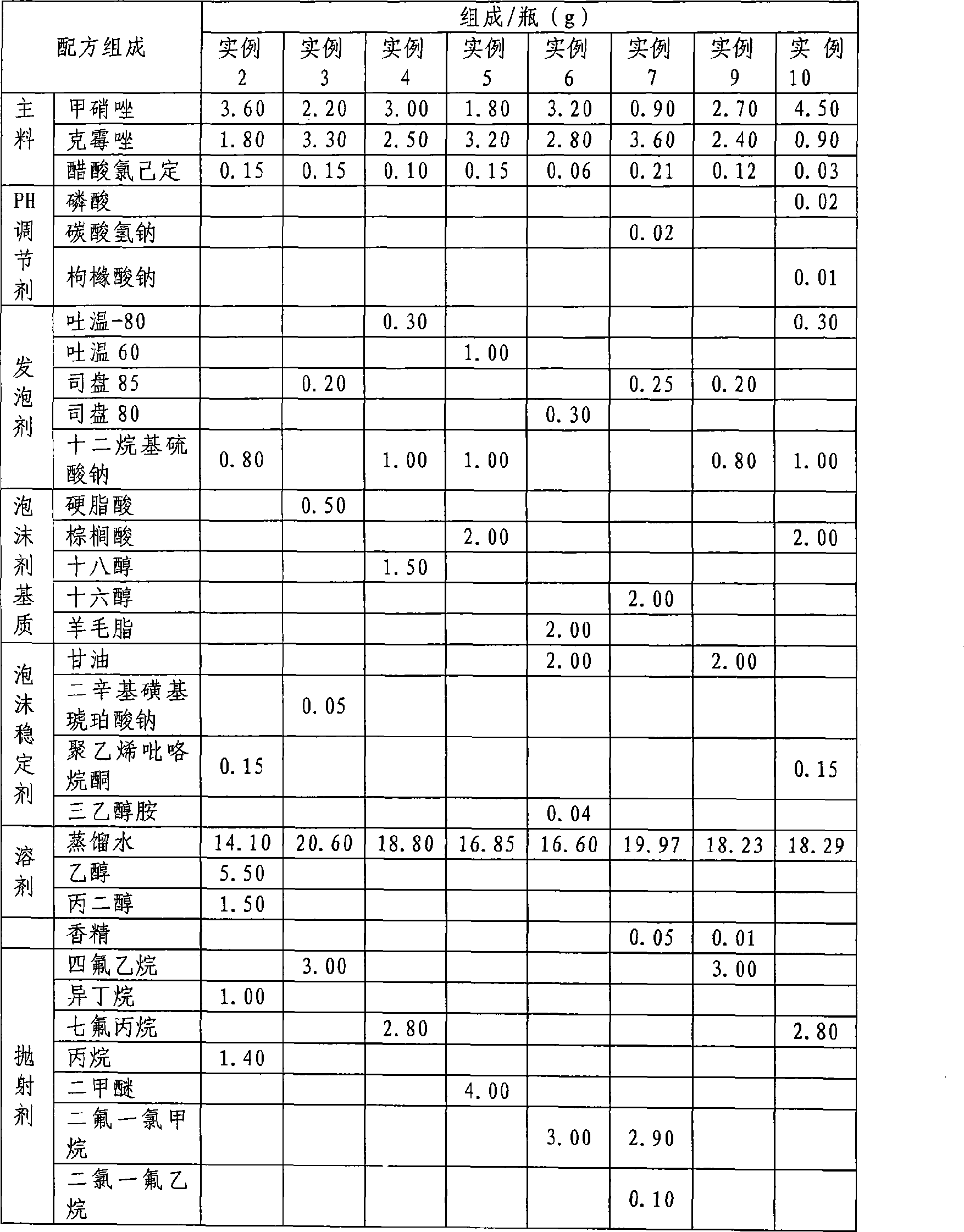
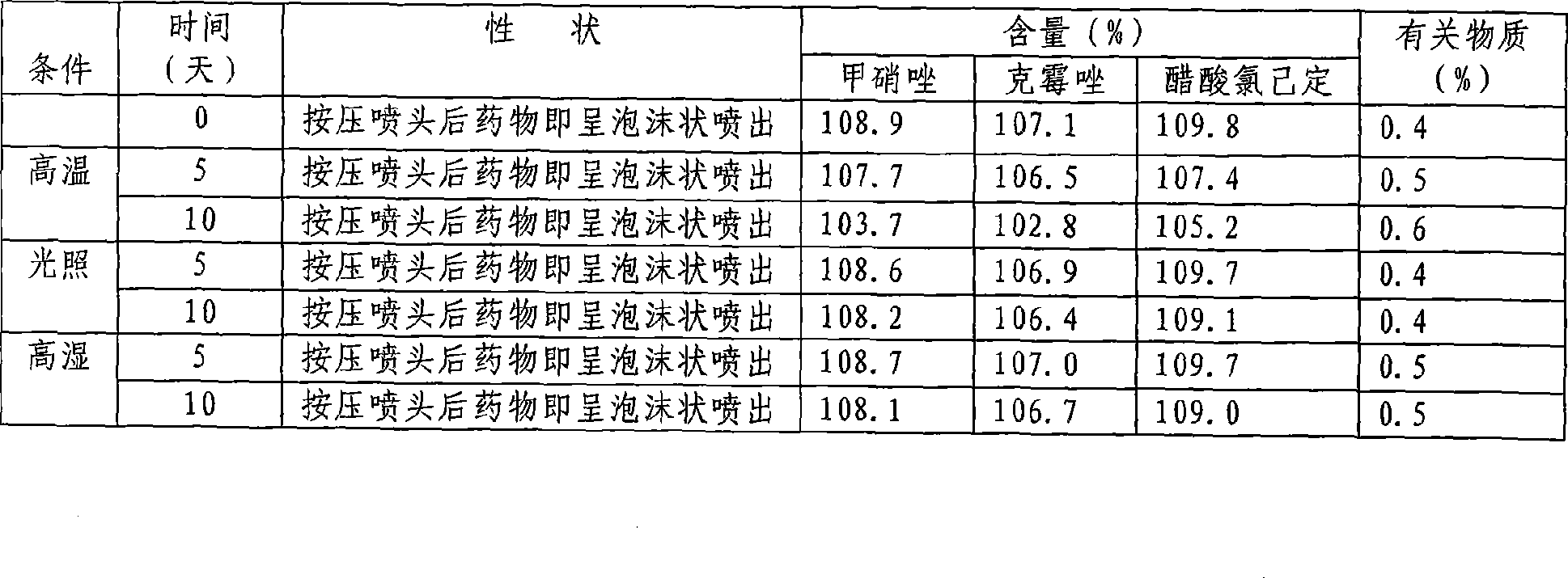
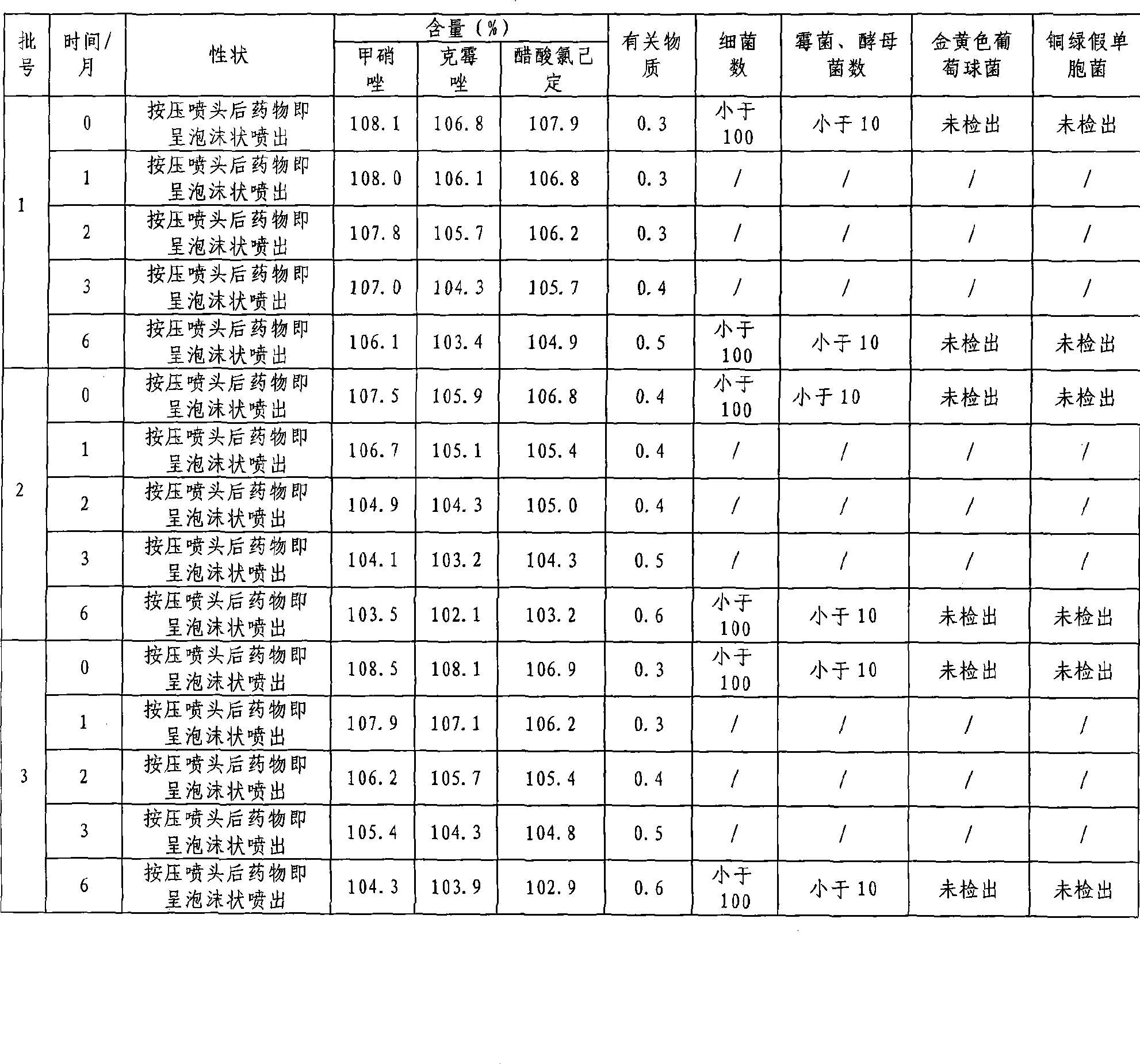
Medicament composition for treating colpitis symptoms and preparation method thereof
ActiveCN101502510AEvenly distributedFast absorptionAntibacterial agentsOrganic active ingredientsChlorhexidine AcetateHigh absorption
The invention relates to a drug combination for curing the inflammation of vagina, which adopts the form of a foaming agent, in particular to a foaming agent with the principle agents being metronidazole, clotrimazole and chlorhexidine acetate. The drug combination has the advantages of even distribution, wide coating area and better effect in the vagina by adopting the form, and meanwhile, the drug combination feels more comfortable and has stronger retention property than other forms. In the formula of the foaming agent, the drug combination can permeate into the mucosal folds of the vagina rapidly and effectively by dispersing atomizing particles with the particle diameter being a few micrometers in a foaming manner under the action of pressure; the drug combination has higher absorption rate than the spraying agent with water being the only solvent with the assistance from various fat-soluble auxiliary materials; the drug contamination and the cross contamination are avoided during the administration; the invention has the advantages that the curative effect is fully produced, the administration is sanitary, convenient, safe and reliable without causing the foreign body sensation, and the drug combination is easily accepted by patients; and the invention further has the advantages of drug stability, form stability and long effective duration.
Owner:YANGTAI PHARMA SHANDONG
Medicinal composition solution and preparation method and use thereof
InactiveCN101966146AQuick effectConducive to clinical rescueOrganic active ingredientsNervous disorderArginineAntioxidant
The invention relates to medicinal composition solution. The medicinal composition solution comprises 2-methy-5-imido-benzo-[d][1,3] oxazine [5-b] pyrazole, arginine, an antioxidant and a solvent, wherein the weight ratio of the 2-methy-5-imido-benzo-[d][1,3] oxazine [5-b] pyrazole, the arginine, the antioxidant to the solvent is 1:0.5 to 15:0.1 to 1.5:10 to 400; and the solvent is pharmaceutical water. The medicinal composition has high water dissolubility, and is difficult to degrade under the protection of the antioxidant. The medicinal composition solution has stable injection exposure, has stable effect of inhibiting coloring change, is not hemolytic, does not separate crystal in serum out and is safe to use.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Medicinal composition solution and preparation method and use thereof
InactiveCN101966146BQuick effectConducive to clinical rescueOrganic active ingredientsNervous disorderAntioxidantArginine
The invention relates to medicinal composition solution. The medicinal composition solution comprises 2-methy-5-imido-benzo-[d][1,3] oxazine [5-b] pyrazole, arginine, an antioxidant and a solvent, wherein the weight ratio of the 2-methy-5-imido-benzo-[d][1,3] oxazine [5-b] pyrazole, the arginine, the antioxidant to the solvent is 1:0.5 to 15:0.1 to 1.5:10 to 400; and the solvent is pharmaceuticalwater. The medicinal composition has high water dissolubility, and is difficult to degrade under the protection of the antioxidant. The medicinal composition solution has stable injection exposure, has stable effect of inhibiting coloring change, is not hemolytic, does not separate crystal in serum out and is safe to use.
Owner:QINGDAO HUANGHAI PHARM CO LTD
A kind of medicinal syrup for treating infantile wind-heat and common cold and preparation method thereof
ActiveCN109223930BImprove drug qualityEnsure medication safetyOrganic active ingredientsDispersion deliveryNepetaForsythia
The invention provides a traditional Chinese medicine composition, in particular to a medicinal syrup for treating wind-heat common cold in children, wherein each ml of syrup comprises 0.0024-0.02 mg of β-pinene, 0.0050-0.2 mg of menthol, 0.068-3 mg of continuous Aromain and 0.64-5mg of gardeniside, the syrup raw materials include forsythia, light tempeh, mint, nepeta, gardenia, rhubarb, artemisia, red peony, betel nut, magnolia, skullcap, pinellia, bupleurum , Licorice. The syrup has clear active ingredients and stable quality. In the preparation process, some traditional Chinese medicines are steamed and assisted by microwave extraction, which improves the efficiency and curative effect.
Owner:JUMPCAN PHARMA GRP
A real-time inspection method for capsules using double detection
ActiveCN105241890BImprove drug qualityRemoval in timeOptically investigating flaws/contaminationSortingReal time validationEngineering
The invention relates to a capsule real-time inspection method with double detection, which comprises the following steps: 1) providing a capsule real-time test platform with double detection, which includes a near-infrared detection mechanism, a visible light detection mechanism and a main controller, wherein the near-infrared detection mechanism and the visible light detection mechanism respectively perform defect detection to every capsule and the main controller is respectively connected to the near-infrared detection mechanism and the visible light detection mechanism for determining a capsule rejection strategy according to a defect detection result from the near-infrared detection mechanism and the visible light detection mechanism; and 2) performing inspection with the test platform. According to the method, there are two detection methods are available to detect the capsules, thereby improving reliability of capsule test.
Owner:NANNING FRESH LIFE BIOTECH
A kind of Guizhi Fuling pellet and preparation method thereof
InactiveCN101491606BQuality improvementLarge distribution areaAntipyreticAnalgesicsIrritationFilm coating
The invention discloses a kind of cinnamon twig and tuckahoe pellets, which are coated pellets, including a core and a film coating wrapped around the core, wherein: the core is composed of cinnamon twigs, poria cocos, cortex moutan, and red peony The five raw materials of peach kernel and peach kernel are made with a weight ratio of 1:1:1:1:1, and the diameter of the pellet core is 1.5mm to 2.0mm; the coating material used for the film coating is an alcohol-soluble gastric-soluble film coating pre- Mixture; the weight of the film coating layer is 1.8-2.2% of the weight of the ball core. The Guizhi Fuling pellet has the effects of promoting blood circulation and removing blood stasis, eliminating phlegm and relieving pain, and regulating qi and blood; and the drug quality is stable, the distribution area of the drug on the surface of the gastrointestinal tract is increased, the irritation is small, the bioavailability is high, and the curative effect is good. The invention also discloses a preparation method of the cinnamon twig and poria pellets. The pellets are prepared by a specific process and then coated. The sterilization effect is good, the molding process is stable, and the quality of the prepared medicine is reliable.
Owner:四川禾邦阳光制药股份有限公司
Para-defovir mesylate related substance and application thereof
PendingCN113884611AImprove drug qualityEasy to produceComponent separationGroup 5/15 element organic compoundsCombinatorial chemistryMedicinal chemistry
Owner:XIAN XINTONG PHARM RES CO LTD
Chinese medicinal compound suppository for treating endometritis of livestock and preparation method thereof
InactiveCN101879240BNo antibiotic residue problemAvoid lostSuppositories deliveryPharmaceutical non-active ingredientsBULK ACTIVE INGREDIENTLivestock
Owner:NINGXIA UNIVERSITY
Traditional Chinese medicine composition for treating leucopenia caused by cancer chemotherapy and preparation method thereof
ActiveCN113144019AReached contentReduce contentUnknown materialsConiferophyta medical ingredientsWhite blood cellPharmaceutical medicine
The invention relates to a traditional Chinese medicine composition for treating leucopenia caused by cancer chemotherapy and a preparation method thereof, and belongs to the technical field of traditional Chinese medicine preparations. According to the technical scheme, the traditional Chinese medicine composition is prepared from the following raw material medicines in parts by weight: 15-90 parts of astragalus membranaceus, 10-70 parts of ox horn bone, 15-60 parts of caulis spatholobi, 15-60 parts of hairyvein agrimony and 10-50 parts of pine nodular branch. The astragalus membranaceus is used as the monarch, the ox horn bone is used as the minister, the caulis spatholobi and the hairyvein agrimony are used as the assistant, and the pine nodular branch is used as the minister, which is in line with the theory of traditional Chinese medicine in treating asthenia. The invention also aims to provide a preparation method of the traditional Chinese medicine composition. The traditional Chinese medicine composition can also be added with pharmaceutically acceptable carriers, and the carriers comprise but are not limited to excipients, flavoring agents, filling agents, adhesives, wetting agents, disintegrating agents and lubricating agents. Clinical embodiments show that the traditional Chinese medicine preparation disclosed by the invention is a medicine for treating leucopenia caused by cancer chemotherapy, which is reliable in curative effect, safe and effective, and is worthy of further popularization.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE +1
Non-turning making method of medium-high temperature Daqu
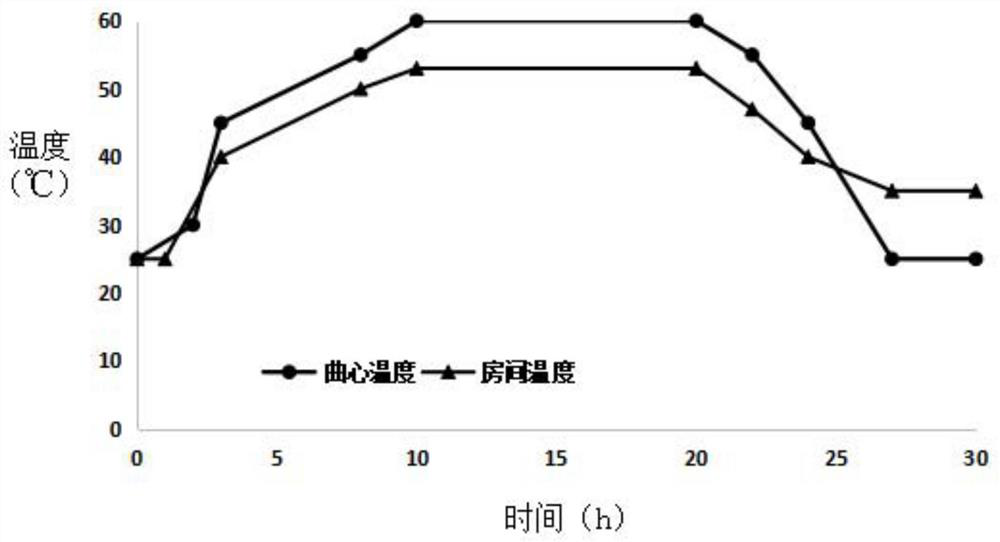
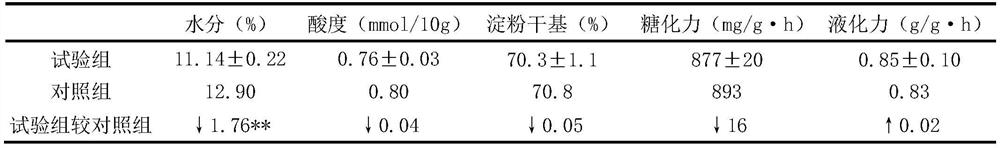
ActiveCN113088415ALiberationLiberation of manual checkingAlcoholic beverage preparationTemperature controlYeast
The invention discloses a Non-turning making method of medium-high temperature Daqu. In a heat preservation yeast room, the pressed yeast blocks are stacked on a frame or a shelf and are uniformly placed in the yeast room, and a fan and an air pipe are introduced; in the fermentation process of Daqu, according to the temperature change of the yeast center, the yeast heating is sequentially divided into six stages, namely a constant room entering stage, a low-temperature heating stage, a medium-temperature heating stage, a high-temperature constant stage, a medium-high-temperature cooling stage and a low-temperature constant stage; wherein the temperature control of each stage is realized by adjusting the frequency of a fresh air valve, the frequency of a fan and an external heat source. Environmental parameters of all positions of the yeast room are adjusted in an air circulation mode, it is guaranteed that environments where yeast blocks are located basically have no difference, and normal fermentation of yeast in the yeast room is achieved.
Owner:JING BRAND +1
Traditional Chinese medicine composition for treating leukopenia caused by cancer chemotherapy and preparation method thereof
ActiveCN113144019BImprove fatigueNo adverse reactionUnknown materialsConiferophyta medical ingredientsWhite blood cellPharmaceutical medicine
The invention relates to a traditional Chinese medicine composition for treating leukopenia caused by cancer chemotherapy and a preparation method thereof, belonging to the technical field of traditional Chinese medicine preparations. The adopted technical scheme is prepared from the following raw materials: 15-90 parts of Astragalus membranaceus, 10-70 parts of ox horn gills, 15-60 parts of Gallatia chinensis, 15-60 parts of Agrimony, and 10-50 parts of turpentine share. In the prescription, Astragalus is the king, ox horn and gills are the ministers, galanthus and Agrimony are the ministers, and the pine knot is the minister, which is in line with the theory of traditional Chinese medicine for treating consumptive labor. Another object of the present invention is to provide a preparation method of the traditional Chinese medicine composition of the present invention. The traditional Chinese medicine composition of the present invention can also be added with pharmaceutically acceptable carriers, which include but are not limited to excipients, flavoring agents, fillers, binders, wetting agents, disintegrating agents and lubricants. Clinical examples show that the traditional Chinese medicine preparation of the present invention is a reliable, safe and effective medicine for treating leukopenia caused by cancer chemotherapy, and is worthy of further promotion.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE +1
Tacrolimus compound and preparation method thereof
ActiveCN106083892BImprove drug qualityHigh purityOrganic chemistryImmunological disordersSolubilityPolymer resin
The invention relates to the technical field of medicine purification, and discloses a highly-pure tacrolimus compound and a preparation method thereof. The method comprises the following steps: preprocessed crude tacrolimus undergoes silver-containing polymer resin column chromatography elution, the above obtained eluate is concentrated, and the above obtained concentrate is re-crystallized to obtain highly-pure tacrolimus. The method has the advantages of simplicity in large-scale production operation, small use amount of a solvent, short production period, low cost and environmental protection; and an HPLC detection result shows that ascomycin and dihydrotacrolimus which are most difficult to separate in the crude tacrolimus are completely removed. The normalization purity of tacrolimus is 99% or above, and obtained tacrolimus crystals have the characteristics of uniform particle size distribution, small internal stress and bulk density, and good stability, fluidity, dispersibility and dissolvability. The quality and the medication safety of tacrolimus are improved.
Owner:HANGZHOU ZHONGMEI HUADONG PHARMA
Cytarabine prodrug related substance and application thereof
PendingCN113621008AImprove drug qualityEasy to produceSugar derivativesComponent separationCytarabineOrganic chemistry
The invention provides a separated compound which is a cytarabine prodrug related substance. The invention also provides application of the compound, and the compound is used for preparing a cytarabine prodrug or a composition containing the cytarabine prodrug.
Owner:XIAN XINTONG PHARM RES CO LTD
A koji-making method for medium-high temperature Daqu without turning koji
ActiveCN113088415BStabilize the initial rate of fermentationQuality improvementAlcoholic beverage preparationTemperature controlFresh air
The invention discloses a koji-making method for medium-high temperature Daqu without turning the koji. In the heat preservation room, the pressed pieces are placed on the frame or shelf, evenly placed in the room, and fans and air ducts are introduced. During the fermentation process of Daqu, the temperature of Daqu is divided into constant temperature according to the change of the temperature of the core. There are six stages: entry stage, low temperature heating stage, medium temperature heating stage, high temperature constant stage, medium high temperature cooling stage, and low temperature constant stage. The temperature control of each stage is realized by adjusting the frequency of fresh air valve and fan and external heating source. The environmental parameters of each position of the Qufang are adjusted by means of wind circulation, so as to ensure that there is basically no difference in the environment of each Qufang, and to realize the normal fermentation of the Qufang Daqu.
Owner:JING BRAND +1
Chinese angelica root and sophora root pellet, and preparation method thereof
InactiveCN101474248BIncreased surface distribution areaLarge distribution areaGranular deliveryDermatological disorderSolubilityClinical efficacy
The invention discloses an angelica and light yellow sophora root micropill, comprising a pill core and a film coat coating the pill core, wherein, the pill core is prepared by bulk drugs, such as angelica and light yellow sophora root of which the proportion by weight is 1 to 1; during the preparation of the pill core, water is used as a binding agent, and 15 ml to 20 ml of water matches 100g ofmedicine powder; and coating materials of the film coat is gastric solubility film coating premix, and the weight of the film coat is 2 to 3 percent of the weight of the pill core. The medicine containing the bulk drugs, such as the angelica and the light yellow sophora root, is processed into micropills by the specific auxiliary material formula and the coating process; and the moulding process and the medical quality are stable, the distribution area of the medicine on the gastrointestinal tract surface is expanded, and the micropill has low stimulation, high bioavailability and stable and reliable clinical efficacy. The invention also discloses a method for preparing the angelica and light yellow sophora root micropill.
Owner:四川禾邦阳光制药股份有限公司
Crystal of polymyxin b1 sulfate, polymyxin b2 sulfate or their mixture and preparation method thereof
InactiveUS20210283213A1Effectively remove impuritiesImprove drug qualityAntibacterial agentsOrganic chemistry methodsOrganic solventPolymyxin B1
The present invention provides an anhydrous crystal of polymyxin B1 sulfate, polymyxin B2 sulfate or a mixture thereof and a preparation method thereof. The preparation method comprises using an organic solvent to precipitate a solid from a saturated solution of polymyxin B1 sulfate, polymyxin B2 sulfate or a mixture thereof, drying it under vacuum to obtain an anhydrous crystal of polymyxin B1 sulfate, polymyxin B2 sulfate or a mixture thereof.
Owner:HUBEI RUIHAO ANKE MEDICINE TECH DEV CO LTD
Preparation technology of captopril tablets
InactiveCN111297811AImprove drug qualityGood stabilityOrganic active ingredientsPharmaceutical non-active ingredientsBiomedical engineeringTableting
The present invention discloses a preparation technology of captopril tablets. The tablets are prepared from the following components in weight percentages: captopril, a filler, a disintegrant, a lubricant and an adhesive. Weighing, screening and mixing are conducted, compressable auxiliary materials are used and direct tableting is conducted to prepare the captopril tablets. The preparation technology reduces the content of hydrolyzed impurity captopril disulfide in the captopril, improves the dissolution rate, and is simple, cost-saving, good in stability, etc.
Owner:仁和堂药业有限公司
Capsule real-time inspection platform with dual detection
ActiveCN105234094BImprove drug qualityRemoval in timeMaterial analysis by optical meansSortingTime capsuleMaster controller
The invention relates to a real-time capsule checkout platform through double detection. The real-time capsule checkout platform through double detection comprises a near-infrared detecting mechanism, a visible light detecting mechanism and a master controller, wherein the near-infrared detecting mechanism and the visible light detecting mechanism performs defect detection on each capsule, the master controller is connected with the near-infrared detecting mechanism and the visible light detecting mechanism, and a capsule rejecting strategy is determined on the basis of defect detection results of the near-infrared detecting mechanism and the visible light detecting mechanism. By means of the real-time capsule checkout platform through double detection, to-be-checked capsules can be detected through two detecting modes, and the reliability of capsule detection is improved.
Owner:HAINAN BRIGHT FUTURE PHARMA
Real-time capsule checkout platform through double detection
ActiveCN105234094AImprove drug qualityRemoval in timeMaterial analysis by optical meansSortingTime capsuleMaster controller
The invention relates to a real-time capsule checkout platform through double detection. The real-time capsule checkout platform through double detection comprises a near-infrared detecting mechanism, a visible light detecting mechanism and a master controller, wherein the near-infrared detecting mechanism and the visible light detecting mechanism performs defect detection on each capsule, the master controller is connected with the near-infrared detecting mechanism and the visible light detecting mechanism, and a capsule rejecting strategy is determined on the basis of defect detection results of the near-infrared detecting mechanism and the visible light detecting mechanism. By means of the real-time capsule checkout platform through double detection, to-be-checked capsules can be detected through two detecting modes, and the reliability of capsule detection is improved.
Owner:HAINAN BRIGHT FUTURE PHARMA
Lyophilized powder of latamoxef sodium for injection and lyophilization method thereof
PendingUS20220304933A1Quality improvementLess impuritiesPowder deliveryOrganic active ingredientsDrugs solutionLatamoxef
A lyophilized powder of latamoxef sodium for injection and a lyophilization method thereof are provided. The lyophilized powder of latamoxef sodium for injection includes the following raw materials by weight: 20˜40 parts of latamoxef sodium, 8˜15 parts of lyophilized adjuvant, 6˜18 parts of excipient and 30˜55 parts of water; by optimizing a raw material process thereof, the present disclosure prepares lyophilized adjuvant and performs twice drying, so that the raw material has stable quality, less impurities, high purity, less adverse reactions and high efficacy; at the same time, the preparation process is innovated and improved to reduce a preparation temperature and ensure stable quality of a drug solution.
Owner:HAINAN HAILING CHEMIPHARMA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com