Traditional Chinese medicine composition formed by ginkgo biloba extract and application thereof to preparation of Shuxuening parenteral solution
An extract and composition technology, applied in the application field of preparing Shuxuening injection, can solve the problems that the chemical composition is not fully confirmed, the active ingredient, the mechanism of action, pharmacokinetics and adverse reactions are not fully explored. , to achieve the effect of benefiting the quality of medicines, reducing adverse reactions, and facilitating separation and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
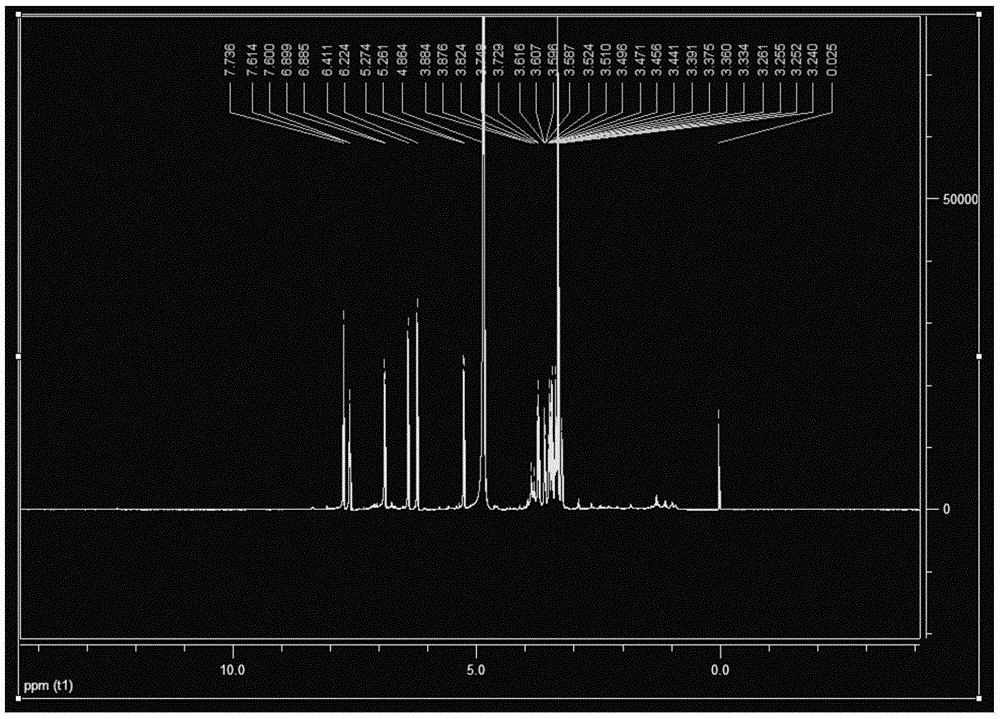
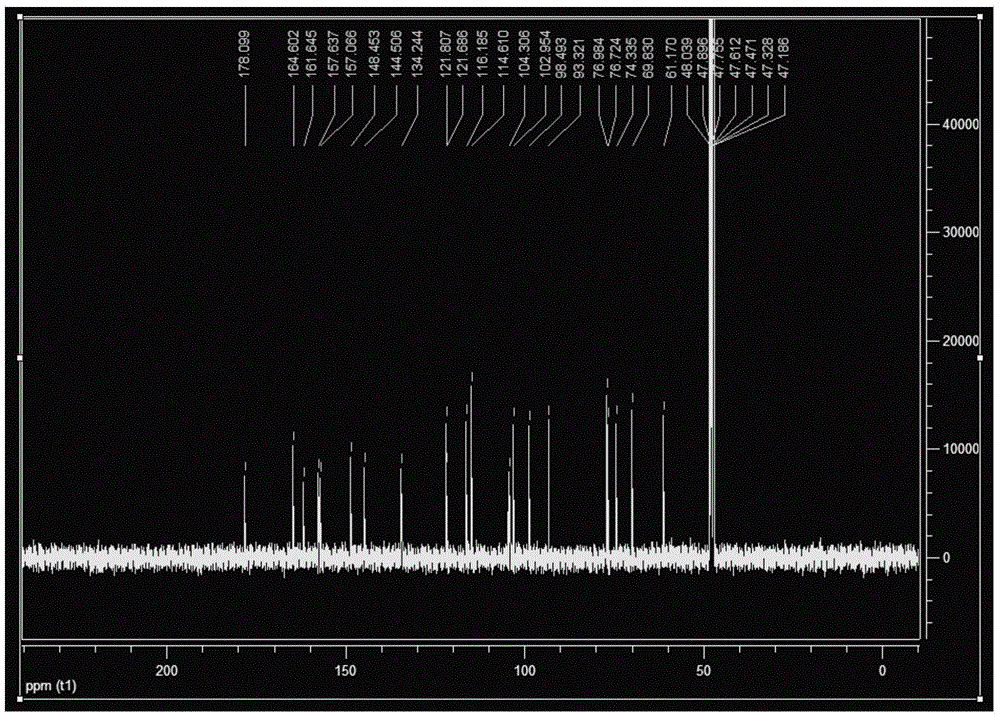
[0046] Preparation of quercetin-3-O-glucoside
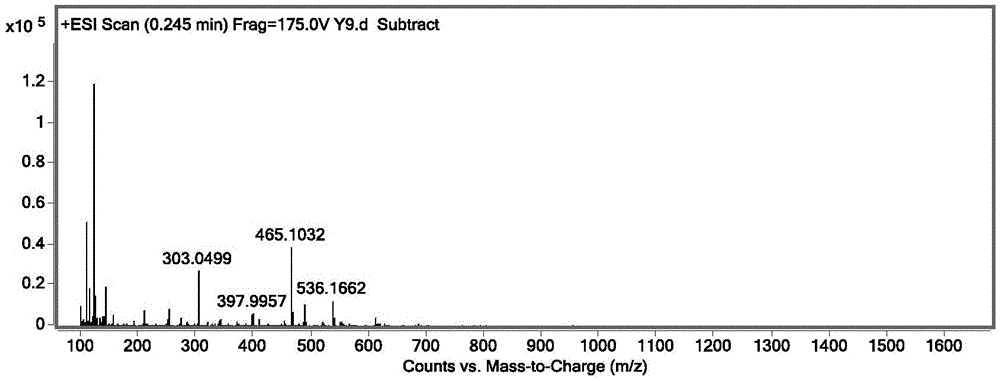
[0047] Weigh 10 g of Ginkgo biloba extract, dissolve it in 50 mL of 40% ethanol-water solution to obtain a Ginkgo biloba extract solution with a concentration of 200 mg / mL, pass through a 0.45 μm microporous membrane, and perform one-dimensional liquid chromatography separation. One-dimensional liquid chromatography adopts silica gel as the matrix of hydrophilic chromatographic filler 50 × 250mm, 10 μm (Huapu New Innovation Technology Co., Ltd.), and the mobile phase adopts methanol containing 0.1% volume concentration of formic acid as the organic phase, containing 0.1% volume The water with formic acid concentration is the water phase, and the gradient elution method: the concentration of the organic phase decreases from 95% to 90% in step gradient in 0-15min. Use DAD ultraviolet detector 360nm to select the absorption wavelength, the preparation temperature is room temperature, the injection volume is 500μL / needle, the mobile ...
Embodiment 2
[0068] Preparation of quercetin-3-O-glucoside
[0069] Weigh 1 g of Ginkgo biloba extract, dissolve it in 100 mL of ethanol-water solution with a volume concentration of 80%, and obtain a Ginkgo biloba extract solution with a concentration of 10 mg / mL, pass it through a 0.45 μm microporous membrane, and perform one-dimensional liquid chromatography. One-dimensional liquid chromatography adopts silica gel-based hydrophilic chromatographic packing (50×250mm, 10μm, Huapu Innovation Technology Co., Ltd.), the mobile phase uses ethanol as the organic phase, water as the aqueous phase, and the concentration of the organic phase is 90 % isocratic, using a DAD ultraviolet detector at 360nm to select the absorption wavelength, the preparation temperature is 40°C, the injection volume is 200μL / needle, the flow rate of the mobile phase is 60mL / min, the components and the remaining components are collected for 15-20 minutes, and the The fractions collected for 15 to 20 minutes were concen...
Embodiment 3
[0078] Preparation of quercetin-3-O-glucoside
[0079] Weigh 100g of Ginkgo biloba extract, dissolve it in 200mL of 50% ethanol-water solution to obtain a Ginkgo biloba extract solution with a concentration of 500mg / mL, pass it through a 0.45μm microporous membrane, and prepare it by one-dimensional liquid chromatography. One-dimensional liquid chromatography adopts a hydrophilic chromatographic packing (50×250mm, 10 μ, Huapu Innovation Technology Co., Ltd.) based on silica gel, and the mobile phase adopts ethanol (containing 0.1% formic acid) as the organic phase, water (containing 0.1% % formic acid) is the aqueous phase, and is eluted by 95% organic equivalence. The DAD ultraviolet detector was used to select the absorption wavelength at 360nm, the preparation temperature was 30°C, the injection volume was 3000 μL / needle, the flow rate of the mobile phase was 120mL / min, the components and the remaining components were collected for 15-20 minutes, and the collected 15 Fract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com