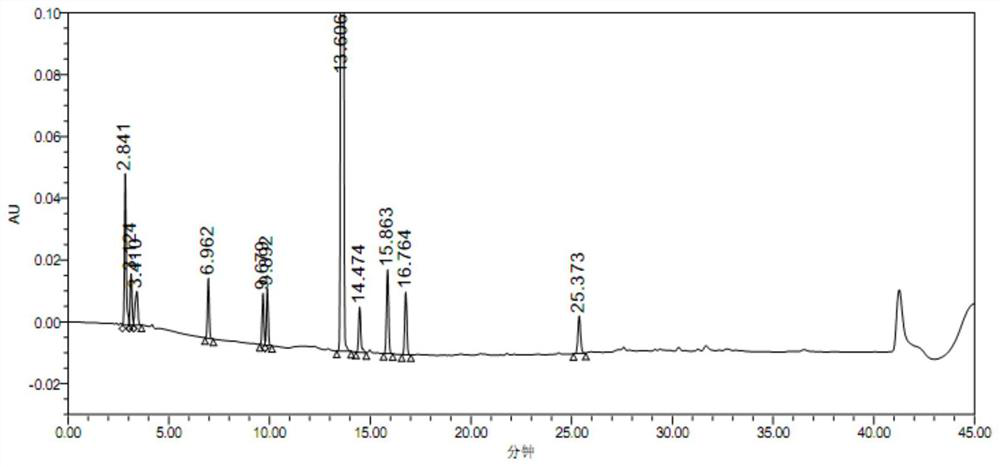
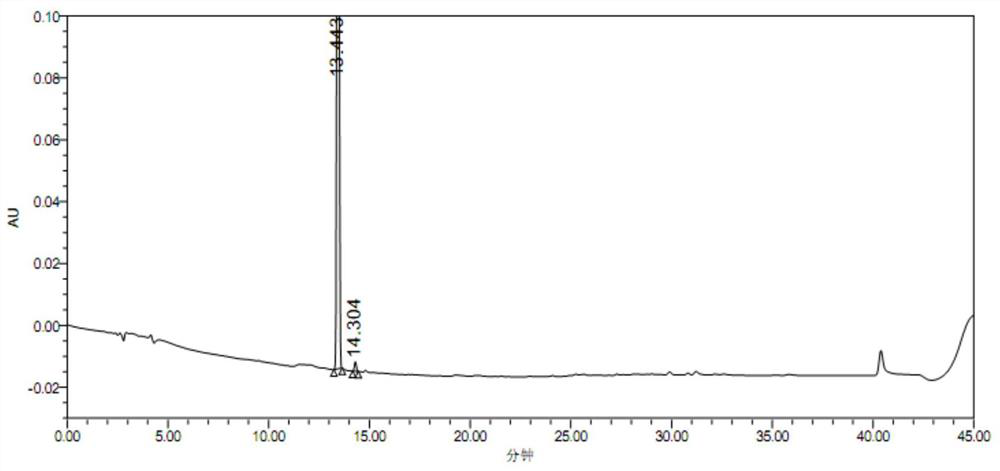
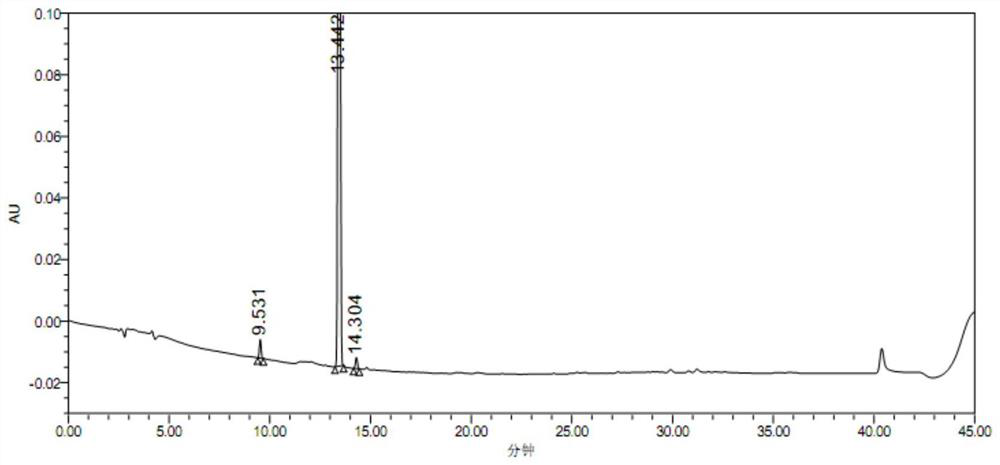
Para-defovir mesylate related substance and application thereof
A related substance, methanesulfonic acid technology, applied in the field of medicine, can solve few problems and achieve the effect of low detection limit, good specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Prescription Composition of Paradefovir Mesylate Tablets
[0105]
[0106] 1) Weighing and preparing materials: dry the silicified microcrystalline cellulose, pregelatinized starch, and silicon dioxide in an oven at 105° C. for 3 hours. Weigh and pulverize paradefovir mesylate, pass through an 80-mesh sieve; mix with silicon dioxide.
[0107] 2) Mixing: the mixed raw material and silicon dioxide mixture are mixed with silicified microcrystalline cellulose and pregelatinized starch for 10 minutes. Add stearic acid again and mix for 8 minutes, discharging.
[0108] 3) Tablet compression: Control the tablet weight according to the content of intermediates, and press plain tablets.
[0109] 4) Coating: the plain tablet is coated into a film-coated tablet.
Embodiment 2
[0111] Phase II clinical trial and preferred dosage
[0112] A randomized, double-blind, positive-drug parallel-controlled, multi-center, phase II dose-finding clinical trial design: with Tenofovir Disoproxil Fumarate Tablets as a control, to evaluate different doses of Paradefovir Mesylate Tablets Effectiveness in the treatment of chronic hepatitis B, evaluation of the safety of different doses of paradefovir mesylate tablets, and exploration of the best safe and effective dose of paradefovir mesylate tablets.
[0113] 240 subjects were divided into 5 groups, respectively 30mg / time, 45mg / time, 60mg / time and 75mg / time of paladefovir mesylate and 300mg / time of tenofovir fumarate Pifufurate positive control group, once a day, orally, the screening period was 14 days, the double-blind test period was 24 weeks, and the observation period was 4 weeks after the course of treatment.
[0114] The main curative effect index is the logarithmic value of serum HBV DNA (PCR method) decrea...
Embodiment 3
[0123] (+)-trans-9-{2-[4-[(S)-(3-Chlorophenyl)-2-oxo-1,3,2-dioxaphosphorin-2-methylene] The preparation of -1-ethyl} adenine mesylate (formula 1):
[0124]
[0125] Add adefovir (29g), NN-diethylformamide (13.2ml), dichloromethane (370ml) into a 1L three-necked flask, and add oxalyl chloride (40ml) dropwise while stirring, the system is white and turbid, and the reaction is refluxed for 2.5h , the system becomes clear. Concentrate at 40°C to obtain yellow semi-solid and semi-liquid. Add dichloromethane (230ml) to dissolve, lower the temperature to below 10°C, add pyridine (10.4ml) in batches, the color changes from brown to dark green, stir for later use, and obtain 2-(6-((diethylamino) Methylamino)-9H-purin-9-yl)ethoxy)methylphosphoryl dichloride.
[0126] Add (S)-1-(3-chlorophenyl)propyl-1,3-diol (20g) and dichloromethane (280ml) to another 1L three-necked flask, lower the temperature to -30°C, and control the temperature < At -20°C, a dichloromethane solution of 2-(6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com