Highly-pure tacrolimus compound and preparation method thereof
A technology of tacrolimus and dihydrotacrolimus, which is applied in the field of high-purity tacrolimus compounds and its preparation, can solve the problems of large amount of elution solvent, complicated purification method, and difficult separation, and achieve the goal of drug administration High safety performance, less solvent consumption, and the effect of improving drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
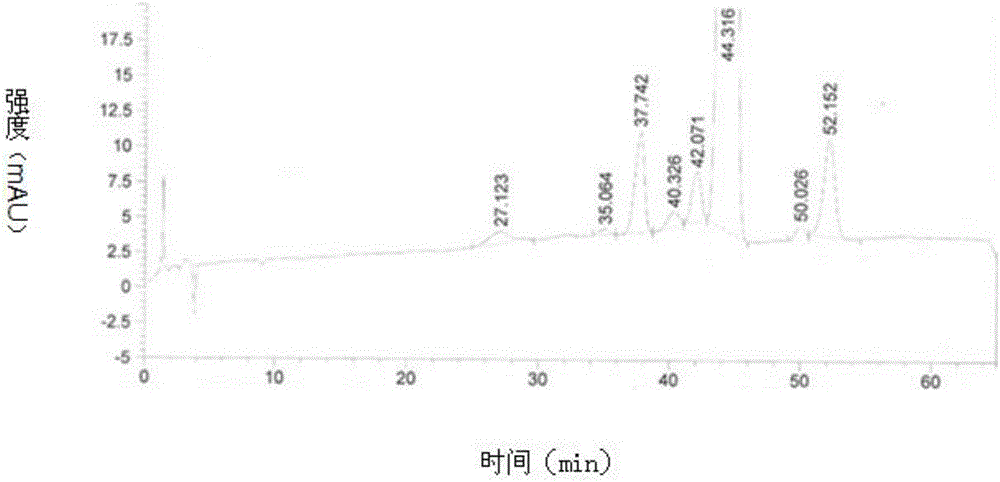
[0060] 1) Take 280ml of silver-ion-containing polymer resin. Before loading the column, put the resin in a glass beaker, add acetone, infiltrate and exceed the resin surface by about 2cm, soak for 3 hours, filter and soak the resin, and put it on the chromatography column Add 1 / 3 volume of acetone, then add the soaked resin, the resin is loaded, the ratio of diameter to height is about 1:7.8; use methanol to regenerate the resin, control the flow rate of 1BV / h, the amount of regenerated acetone is 5BV of the column volume, and then Equilibrate with a mixed solvent with a volume ratio of methanol and ethyl acetate of 49:51, and the amount of the mixed solvent is 2BV of the column volume.
[0061] 2) Preparation and loading of the upper column solution: Calculate the total amount of the upper column (9 mg per ml of chromatography resin) according to the volume of the chromatography resin, and weigh 2.8 g of the crude product of tacrolimus, which contains 1.56% Ascomyces Dihydrot...
Embodiment 2
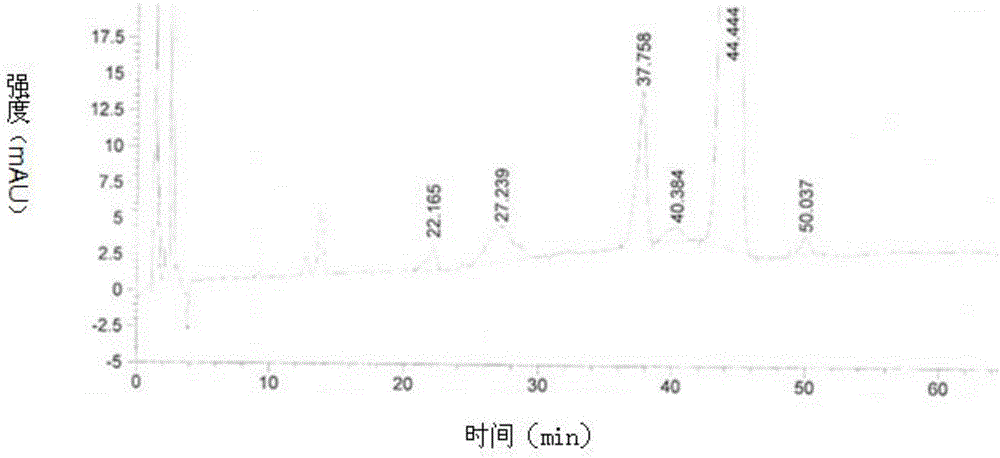
[0066] 1) Take 280ml of silver-ion-containing polymer resin (chromatographic column ¢3.6cm), regenerate the resin with ethanol, control the flow rate of 1BV / h, the amount of regeneration solvent is 2BV of the column volume, and then use ethanol to ethyl acetate volume ratio of 45 : The mixed solvent balance of 55, the mixed solvent consumption is 2BV of column volume.
[0067] 2) Preparation and loading of the upper column solution: calculate the total amount of the upper column (10 mg per ml of chromatography resin) according to the volume of the chromatography resin, and weigh 3.11 g of the crude product of tacrolimus intermediate, which contains 1.56% Ascomycin, 4.11% of dihydrotacrolimus, the weight content is 90%, wherein 2.8g is the active ingredient, dissolved in the mixed solvent that the volume ratio of ethanol and ethyl acetate is 45:55, is made into 250mg / ml of loading solution. Before loading the column, first remove the solvent at the upper end of the chromatogr...
Embodiment 3
[0072] 1) Take 120L silver-ion-containing polymer resin. Before loading the column (chromatographic column ¢30cm), the resin needs to be placed in a stainless steel barrel, and ethanol is added to infiltrate and exceed the surface layer of the resin by 2cm. After soaking for 4 hours, filter Soak the resin, add 1 / 3 medium polar solvent into the chromatography column, and then add the soaked resin. After the resin is loaded, the ratio of diameter to height is about 1:5.7; use ethanol to regenerate the resin, control the flow rate of 1BV / h, and regenerate The amount of solvent is 5BV of the column volume, and then equilibrated with a mixed solvent with a volume ratio of ethanol:ethyl acetate of 45:55, and the amount of mixed solvent is 5BV of the column volume.
[0073] 2) Preparation and loading of the upper column solution: Calculate the total amount of the upper column (9 mg per ml of chromatography resin) according to the volume of the chromatography resin, and weigh 1220 g of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Potency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com