Separation and/or detection method of impurities in atorvastatin calcium
A technology of atorvastatin calcium and atorvastatin is applied in the field of separation or detection of atorvastatin calcium enantiomer and atorvastatin calcium lactone, which can solve the problem that the accuracy of detection results cannot be guaranteed , can not be effectively separated and other problems, to improve the quality and safety of drugs, the effect of good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Testing instruments and chromatographic conditions
[0068] Detection instrument: high performance liquid chromatography
[0069] Chromatographic conditions: the chromatographic column is a chiral chromatographic column of polysaccharide derivatives, the column temperature is 35°C, an ultraviolet detector is used, and the detection wavelength is set to 244nm; the flow rate of the mobile phase is 1ml / min; the volume ratio is 850:150: 1 of n-hexane-absolute ethanol-trifluoroacetic acid is the mobile equal degree elution.
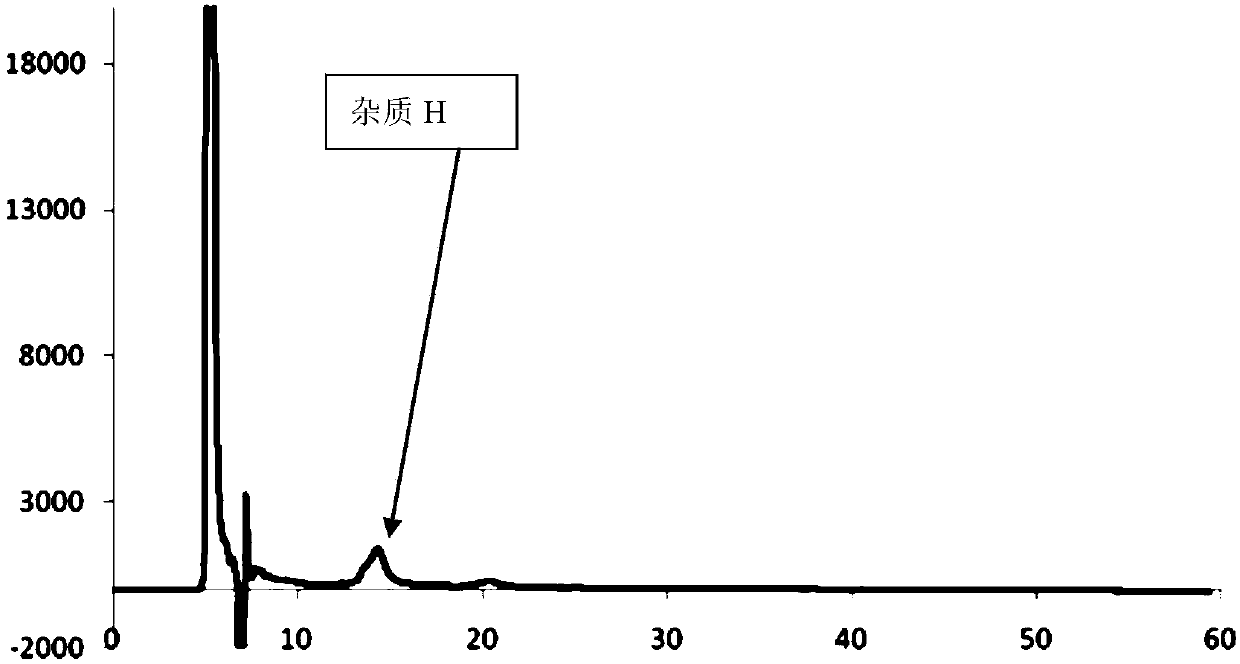
[0070] Preparation of atorvastatin calcium impurity H positioning solution: Weigh an appropriate amount of atorvastatin calcium impurity H, add solvent to dissolve and dilute to the scale, so that the concentration of atorvastatin calcium impurity H is 0.002mg / mL, filter, and the solvent Methanol-absolute ethanol-n-hexane with a volume ratio of 1:1:3;
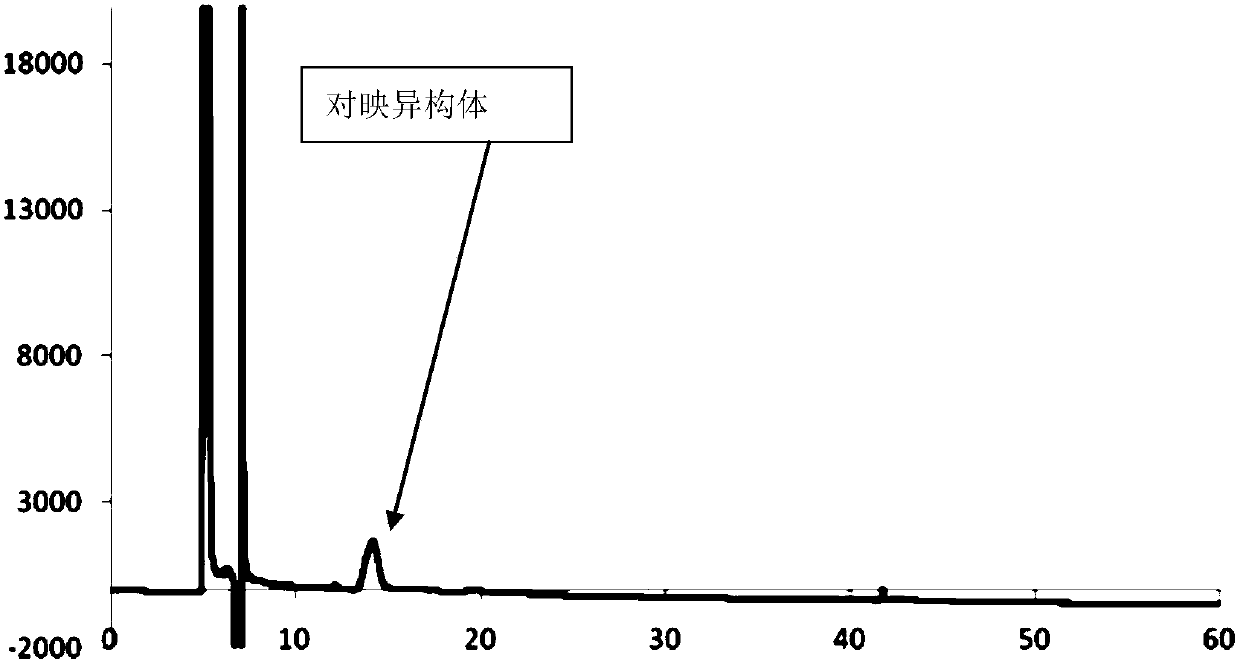
[0071] Preparation of atorvastatin calcium enantiomer localization solution: Weigh an appropriate a...
Embodiment 2
[0078] Testing instruments and chromatographic conditions
[0079] Detection instrument: high performance liquid chromatography
[0080] Chromatographic conditions: the chromatographic column is a chiral chromatographic column of polysaccharide derivatives, the column temperature is 35°C, an ultraviolet detector is used, and the detection wavelength is set to 244nm; the flow rate of the mobile phase is 1ml / min; the volume ratio is 95:2: 3: 0.2: 0.2 n-hexane-methanol-absolute ethanol-formic acid-diethylamine is the mobile equal degree elution.
[0081] Blank excipient solution: add atorvastatin calcium blank excipient to the solvent accounting for 50% of the total volume of the test sample solution, shake fully to dissolve atorvastatin, add solvent to constant volume, so that the concentration of blank excipient is equivalent to that of atorvastatin Statin calcium 1 mg / mL, filtered, wherein the solvent is methanol-absolute ethanol-n-hexane with a volume ratio of 1:1:3.
[008...
Embodiment 3
[0087] Testing instruments and chromatographic conditions
[0088] Detection instrument: high performance liquid chromatography
[0089] Chromatographic conditions: the chromatographic column is a chiral chromatographic column of polysaccharide derivatives, the column temperature is 35°C, an ultraviolet detector is used, and the detection wavelength is set to 244nm; the flow rate of the mobile phase is 1ml / min; the volume ratio is 95:2: 3: 0.1:0.1 n-hexane-methanol-absolute ethanol: trifluoroacetic acid: triethylamine for mobile equipotential elution.
[0090] The preparation of the system suitability solution in this example is the same as in Example 1.
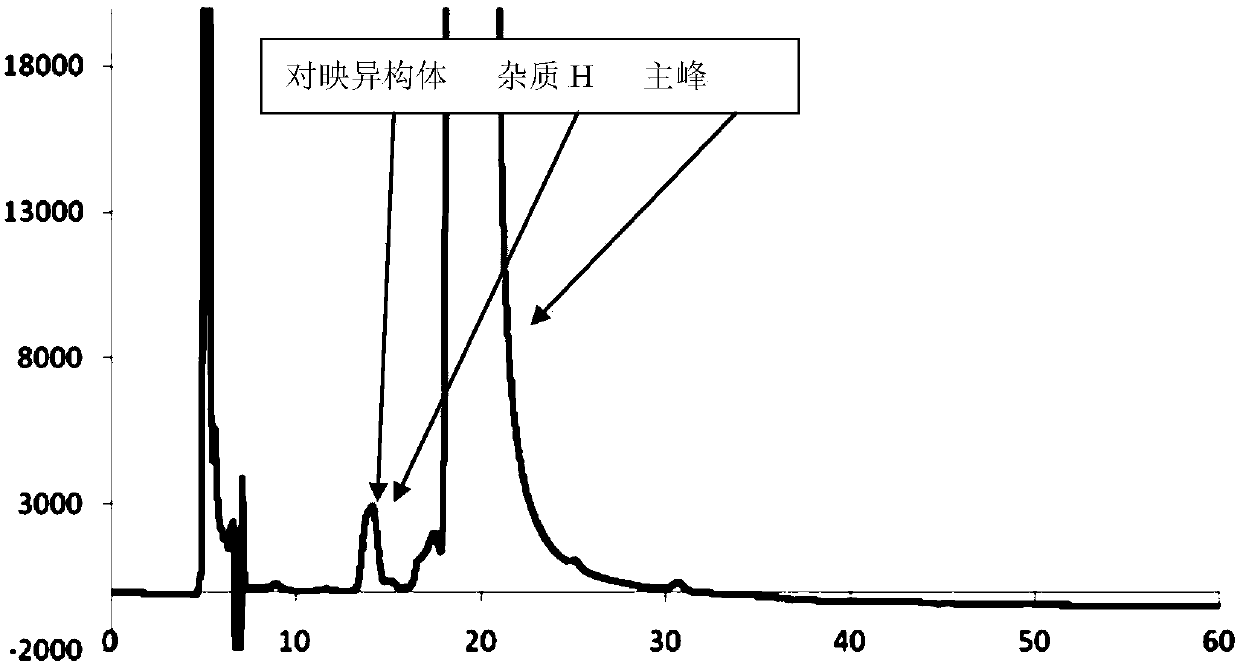
[0091] Take 20 μl of the system suitability solution, inject it into the liquid chromatograph, measure it according to the chromatographic conditions, record the chromatogram, and complete the determination; the obtained chromatogram is as follows: Figure 7 shown.
[0092]The results showed that: atorvastatin calcium ena...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com