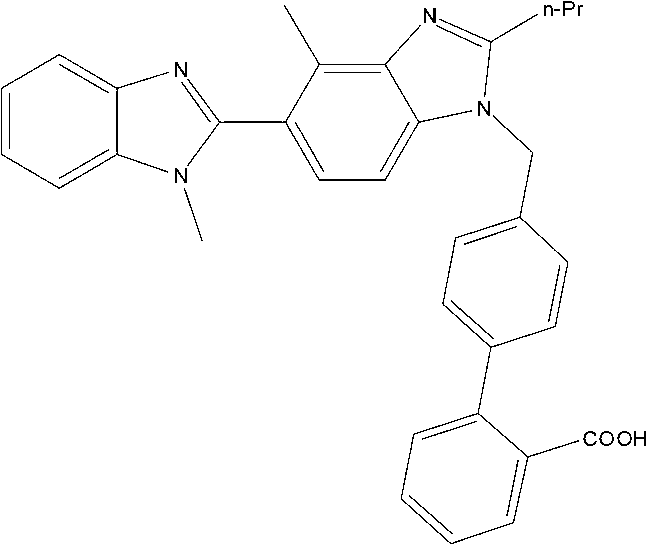
Method for preparing telmisartan
A technology for telmisartan and a compound, which is applied in the field of preparation of antihypertensive drugs, can solve the problems of being unfavorable to industrialized production, cumbersome operation, low overall yield and the like, and achieves remarkable economic benefits, reasonable route and improved yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 prepares telmisartan
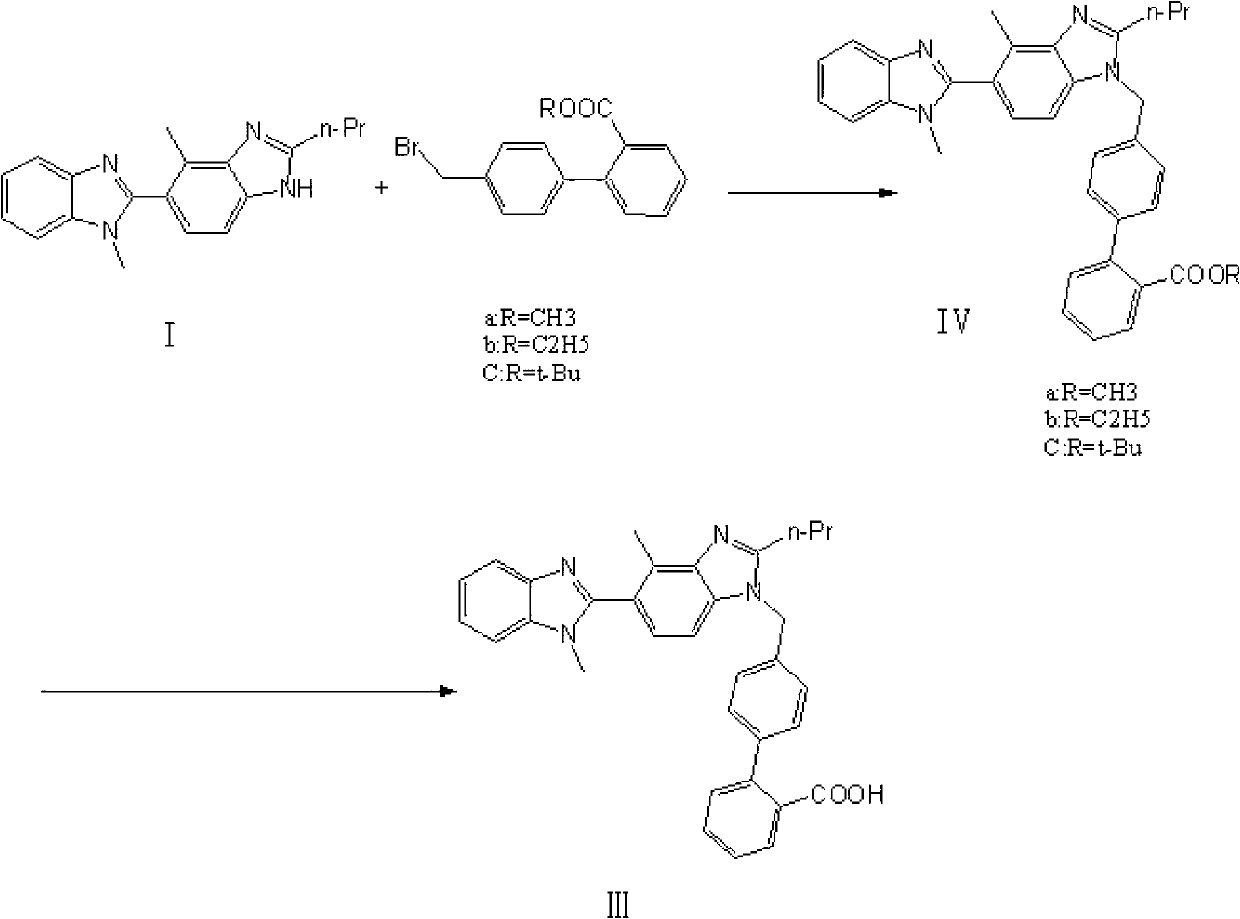
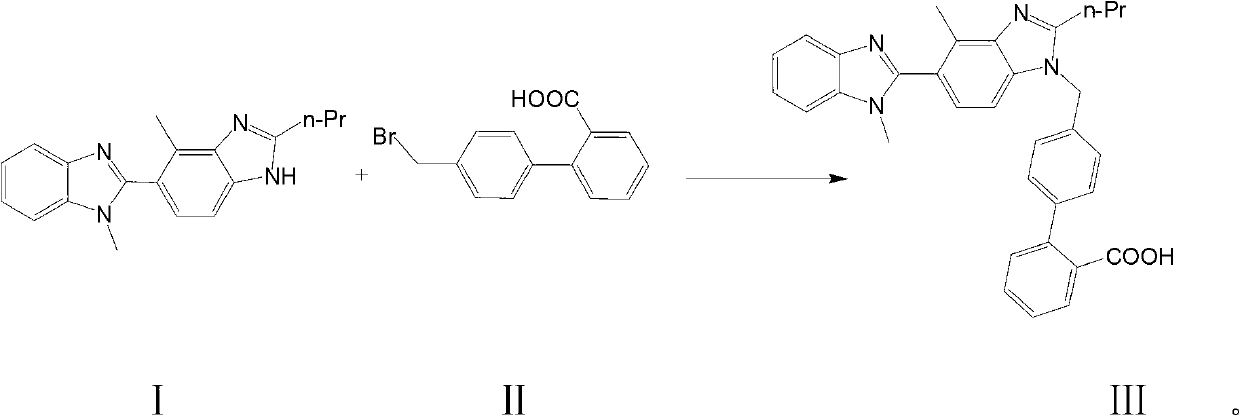
[0022] 2-n-propyl-4-methyl-6-(1'-methylbenzimidazol-2-yl)benzimidazole (30.4g, 0.10mol) shown in formula I, 4-bromomethylbi Benzene-2-carboxylic acid (43.6g, 0.15mol), potassium carbonate fine powder (30g, 0.2mol) mixed with DMSO 500ml, reacted at 100°C for 10 hours, poured the reaction solution into ice water, and slowly adjusted to pH2-3, solid precipitated. Filter and dry at 70°C to obtain a crude product, which is heated and beaten once with 300 ml of ethyl acetate. Filter and dry at 70°C. Recrystallized from DMF to obtain 44.2 g of telmisartan represented by formula III, yield: 86.5%.
[0023] Wherein, the DMSO can be replaced by any one of DMF, dioxane, pyrrolidones, butanone, 2-methylisobutyl ketone and ethylene glycol dimethyl ether.
Embodiment 2
[0024] Embodiment 2 prepares telmisartan
[0025] 2-n-propyl-4-methyl-6-(1'-methylbenzimidazol-2-yl)benzimidazole (30.4g, 0.10mol) shown in formula I, 4-bromomethylbi Benzene-2-carboxylic acid (37.83g, 0.13mol), potassium hydroxide (5.6g, 0.10mol) and 500ml of 2-methylisobutyl ketone were mixed, reacted at 80°C for 16 hours, and poured the reaction solution into ice water , Slowly adjust the pH to 2-3 with dilute hydrochloric acid, and precipitate a solid. Filter and dry at 70°C to obtain a crude product, which is heated and beaten once with 300 ml of ethyl acetate. Filter and dry at 70°C. Recrystallized from DMF to obtain 38.8 g of telmisartan represented by formula III, yield: 76%.
[0026] Wherein, the 2-methylisobutyl ketone can be replaced by any one of DMF, DMSO, dioxane, pyrrolidones, butanone and ethylene glycol dimethyl ether.
Embodiment 3
[0027] Embodiment 3 prepares telmisartan
[0028] 2-n-propyl-4-methyl-6-(1'-methylbenzimidazol-2-yl)benzimidazole (30.4g 0.10mol) shown in formula I, 4-bromomethylbiphenyl -2-Carboxylic acid (43.6g, 0.15mol), sodium ethoxide (13.6g, 0.2mol) mixed with 500ml of N-methylpyrrolidone, reacted at 50 degrees Celsius for 10 hours, poured the reaction solution into ice water, and slowly Adjust the pH to 2-3 and precipitate a solid. Filter and dry at 70°C to obtain a crude product, which is heated and beaten once with 300 ml of ethyl acetate. Filter and dry at 70°C. Recrystallized from DMF to obtain 27.7 g of telmisartan represented by formula III, yield: 54%.
[0029] Wherein, the N-methylpyrrolidone can be replaced by any one of DMF, DMSO, dioxane, other pyrrolidones, 2-methylisobutyl ketone, butanone and ethylene glycol dimethyl ether.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com