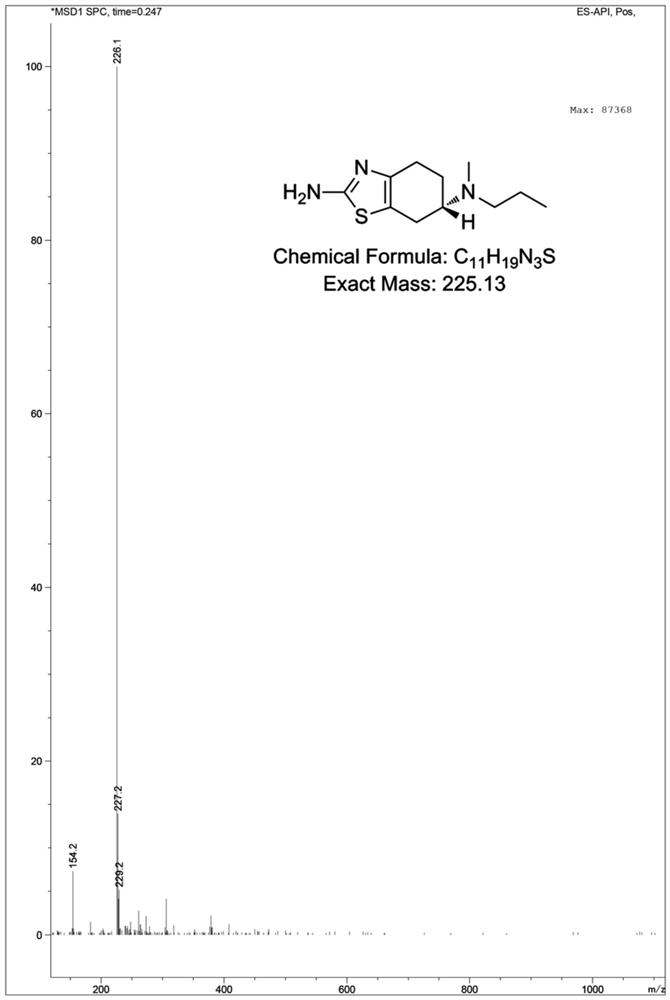
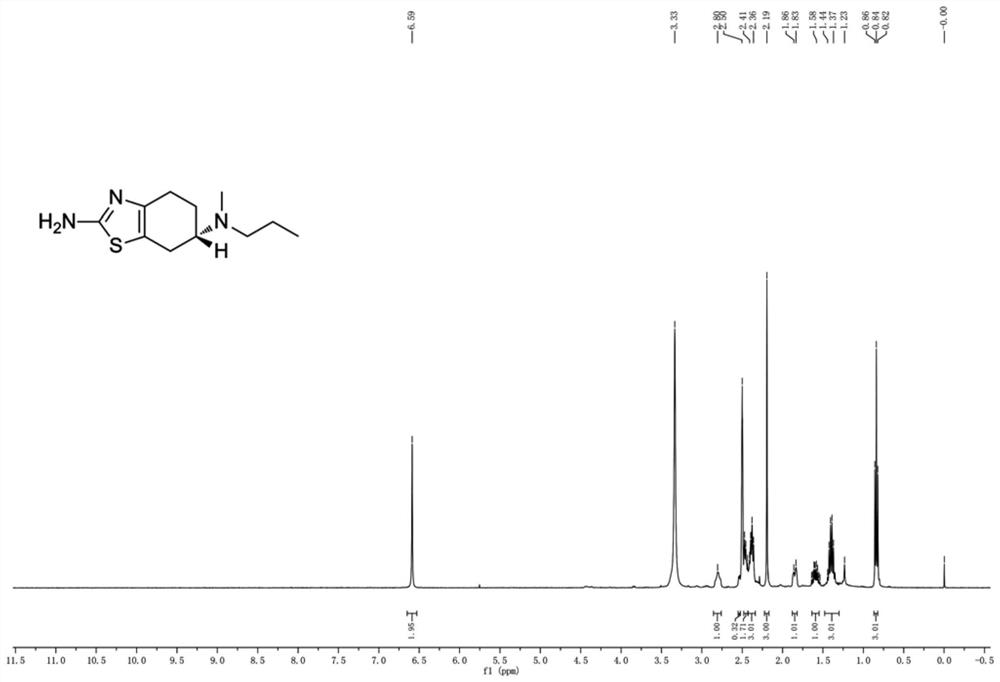
Preparation method of pramipexole N methyl impurity
A pramipexole and methyl technology, applied in the field of preparation of pharmaceutical impurity standard products, can solve the problems of less by-products, shortage of reference substances, etc., and achieve the effects of less by-products, convenient operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of intermediate compound of formula 2
[0031] The compound of formula 1 (0.5 g, 2.37 mmol, 1 eq), N,N-diisopropylethylamine (1.173 mL, 7.10 mmol, 3 eq) and dichloromethane (20 mL) were added to the three-necked flask, and the mixture was stirred and dissolved at room temperature. After adding Boc 2 O (0.54 mL, 2.3 mmol, 1 eq). The reaction was then continued to stir for 12 hours, and TLC monitored 1 to complete the reaction. Add 100 mL of water, extract 3 times with dichloromethane (30 mL), combine the organic phases, use anhydrous Na 2 SO 4 Dry, filter, concentrate the filtrate to remove the solvent, and purify by silica gel column chromatography. The elution conditions are: dichloromethane:methanol=15:1 to obtain 0.61 g of the intermediate compound of formula 2 as a white solid with a yield of 83%.
Embodiment 2
[0032] Embodiment 2: the preparation of pramipexole N methyl impurity
[0033] Under nitrogen protection, the intermediate 2 compound (0.18 g, 0.59 mmol, 1 eq) dissolved in anhydrous toluene (7.2 mL) was added to the three-necked flask, then cooled to 0 °C, and LAH (lithium aluminum hydride) was slowly added in batches ( 0.23 g, 5.9 mmol, 10 eq). Then return to room temperature and stir for 3 hours, and then the temperature is raised to 110° C. to react overnight. The next day, LC-MS detected that the reaction was complete, the reaction solution was lowered to 0 °C, and then diethyl ether (10 mL), H 2 O (0.3 mL), continued dropwise addition of 5% NaOH (0.5 mL) and H at this temperature 2 O (1 mL), finally add anhydrous Na 2 SO 4 , then filtered, the filtrate was concentrated to remove the solvent, and the residue was purified by silica gel column chromatography with the elution conditions: MeOH:DCM:NH 4 OH=1:9:0.1 to get pramipexole N methyl impurity 0.118 g, the yield is...
Embodiment 3
[0043] Example 3: Preparation of intermediate compound of formula 2
[0044] The compound of formula 1 (0.50 g, 2.37 mmol, 1 eq), triethylamine (0.98 mL, 7.10 mmol, 3 eq) and tetrahydrofuran (10 mL) were added to the three-necked flask, and after stirring at room temperature to dissolve, Boc was added. 2O (0.60 mL, 2.61 mmol, 1.1 eq). Then continue to stir and react for 12 hours, TLC monitoring 1 reaction is complete. Add 100 mL of water, extract 3 times with dichloromethane (30 mL), combine the organic phases, wash with anhydrous Na 2 SO 4 After drying and filtering, the filtrate was concentrated to remove the solvent, and purified by silica gel column chromatography under the elution condition: dichloromethane:methanol=15:1 to obtain 0.63 g of the intermediate compound of formula 2 as a white solid with a yield of 85.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com