Biomarker combination for diagnosing non-obstructive azoospermia and application thereof
A biomarker, azoospermia technology, applied in the field of medical diagnosis, can solve problems such as patient pain, achieve the effect of alleviating pain, overcoming technical obstacles, and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The construction of embodiment 1 mouse NOA model
[0023] Methods: Sexually mature male mice weighing 25-35 g were used. Dissolve busulfan in DMSO, dilute it with normal saline to obtain the dilution, disinfect the lower abdomen of the mouse with alcohol, and after drying, absorb the dilution of busulfan and inject it into the mouse intraperitoneally. After 3 injections, 35-37 days later, the mouse model of azoospermia was obtained. After sacrificing the mice, the whole blood of the mice was taken for ELISA (enzyme-linked immunosorbent assay), and the testicular tissues of the mice were taken for WB, H&E and immunofluorescence staining to test the accuracy of the mouse whole blood ELISA.
Embodiment 2E
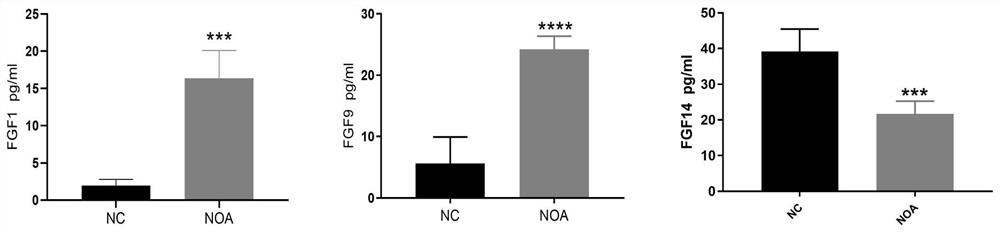
[0024] Example 2 ELISA method detects protein expression analysis of mouse NOA model blood samples
[0025] After the NOA model was established, the levels of FGF1, FGF9, and FGF14 in the serum of mice were detected by enzyme-linked immunosorbent assay kit (ELISAKit).
[0026] The operation steps of ELISA detection are as follows:
[0027] (1) Add 50 μl of FGF1, FGF9, FGF14 standard substances, serum samples and reference substances to the 96-well plate coated with the antibody, the reference substance is the blank control substance in the kit, that is, the sample diluent, Used to remove background absorbance differences caused by reagents.
[0028] The standard substance is the standard substance with different concentrations given in the kit, which is used to prepare the standard curve.
[0029] The preparation method of the serum sample is as follows: after removing eyeballs from NOA model mice, centrifuging at 3000r for 15 minutes at 4°C for 15 minutes, taking the supern...
Embodiment 3WB
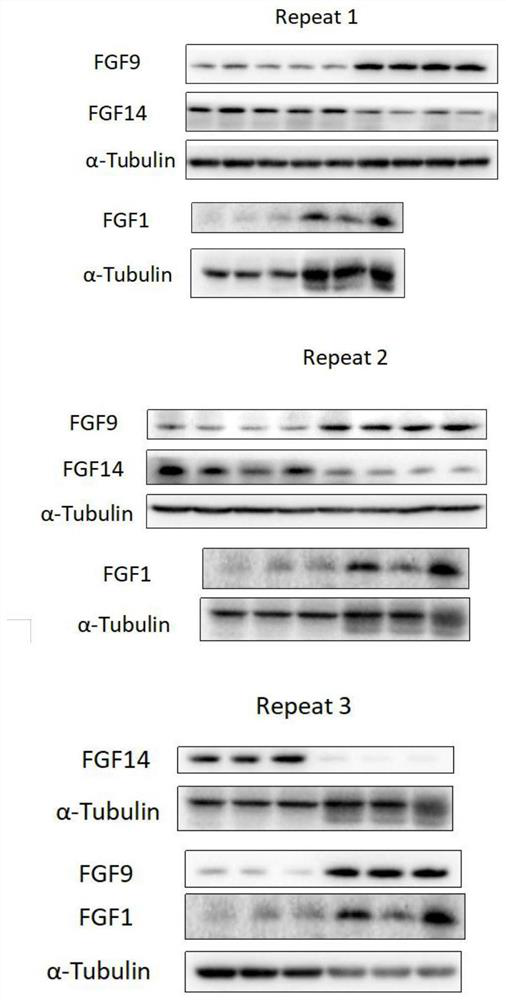
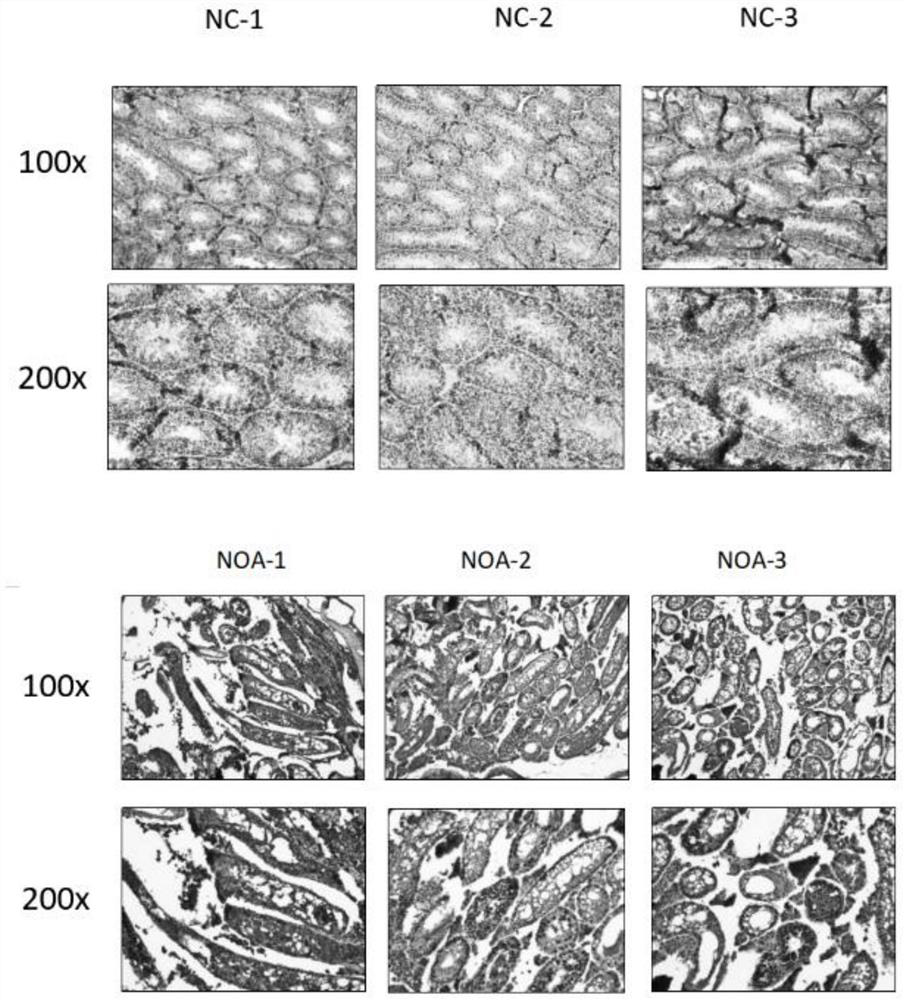
[0038] Example 3WB method, H&E method and immunofluorescence staining method detect the protein expression analysis of the mouse NOA model testis tissue sample
[0039] In order to further verify the results of protein analysis in NOA model serum, blood was collected and testicular tissues of model mice were taken for protein expression analysis by WB, H&E and immunofluorescence staining. The specific operation steps are as follows:
[0040] 1. Western blot operation steps are as follows:
[0041](1) Extraction of total protein: Take 0.05 g of NOA model mouse testis tissue, add 0.5 ml of mammalian tissue protein extraction reagent containing PMSF (extraction reagent: PMSF=100:1), and homogenize to Evenly organized. After standing on ice for 15 minutes, place it at 4°C and centrifuge at 12,000 rpm for 15 minutes, and transfer the supernatant to an EP tube.
[0042] (2) Determination of protein concentration by BCA method: Take 0, 1, 2, 4, 8, 12, 16, 20 μl of 0.5 mg / ml standa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com