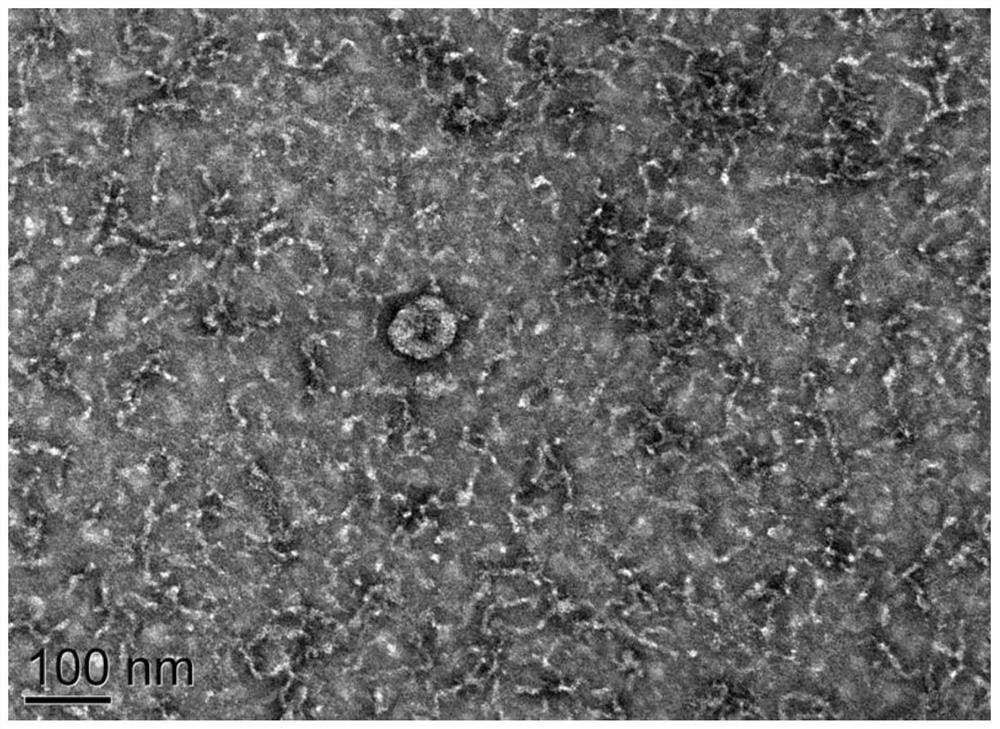
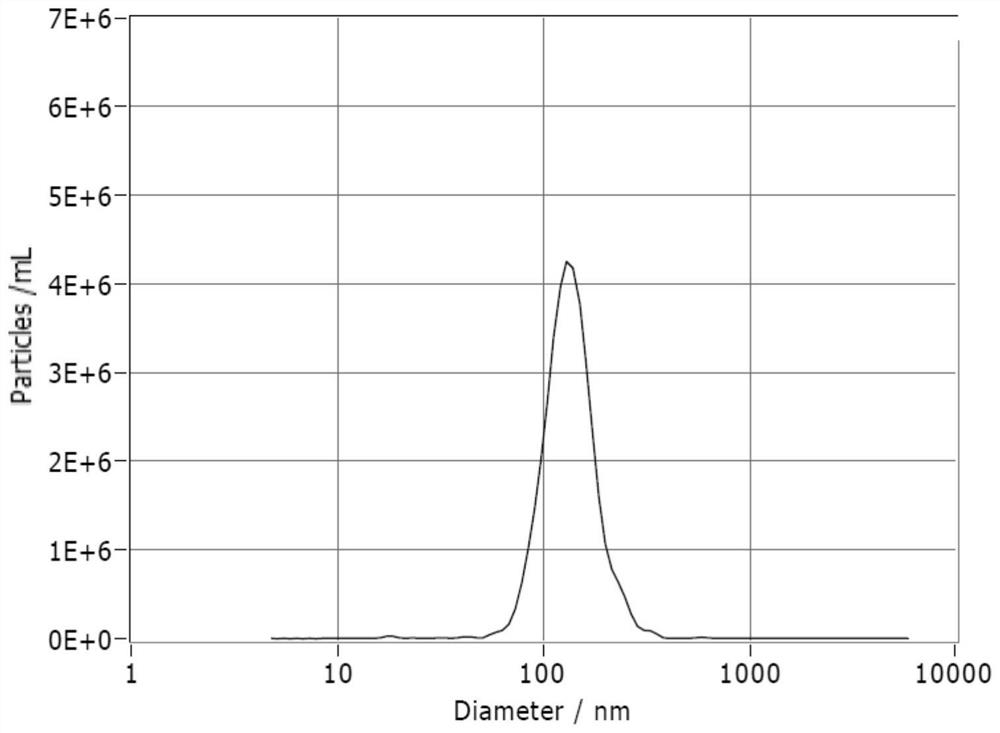
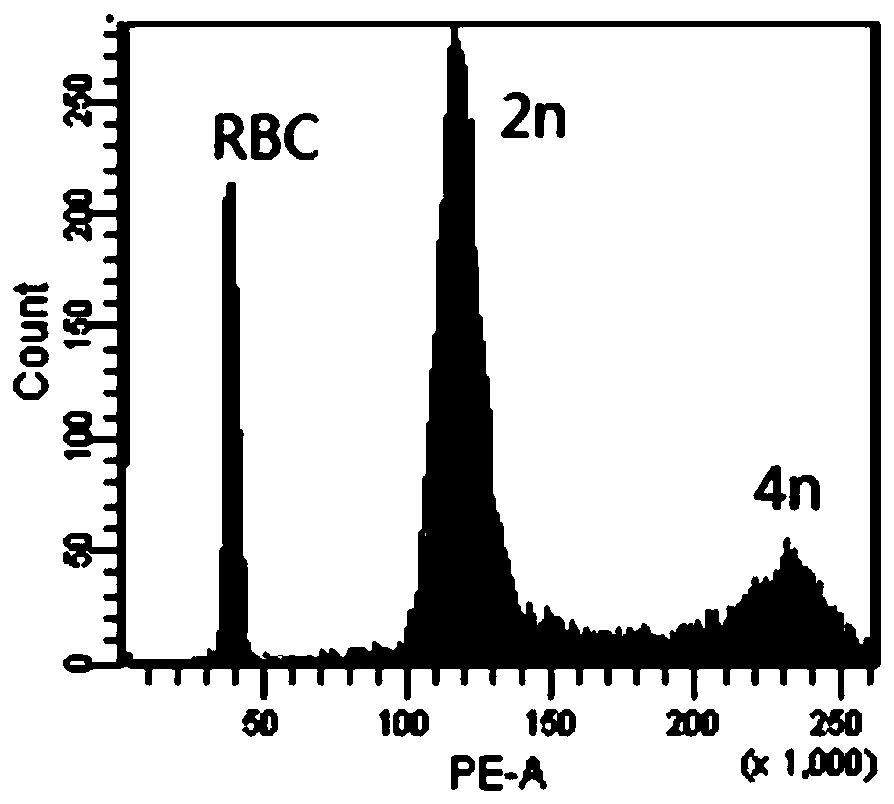
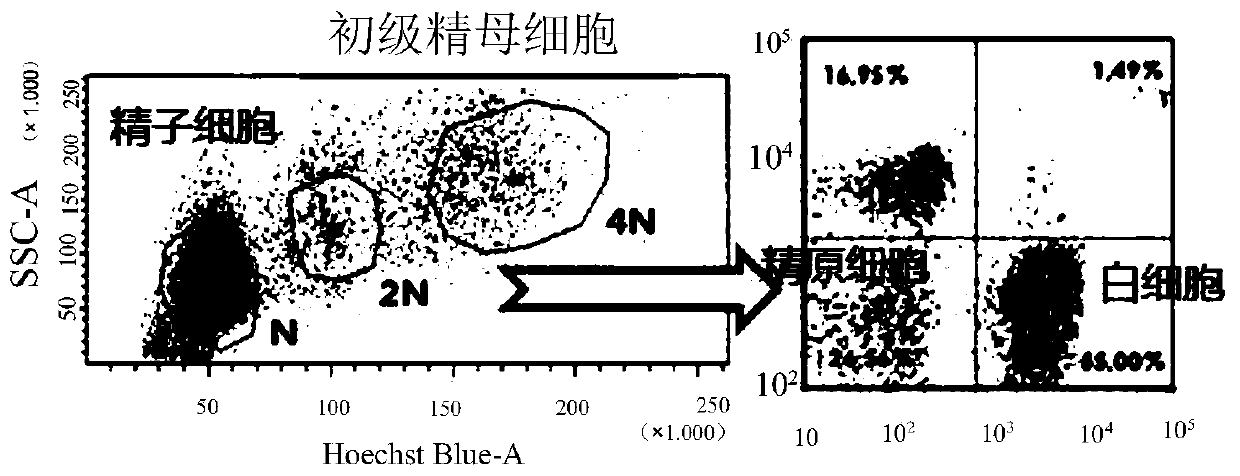
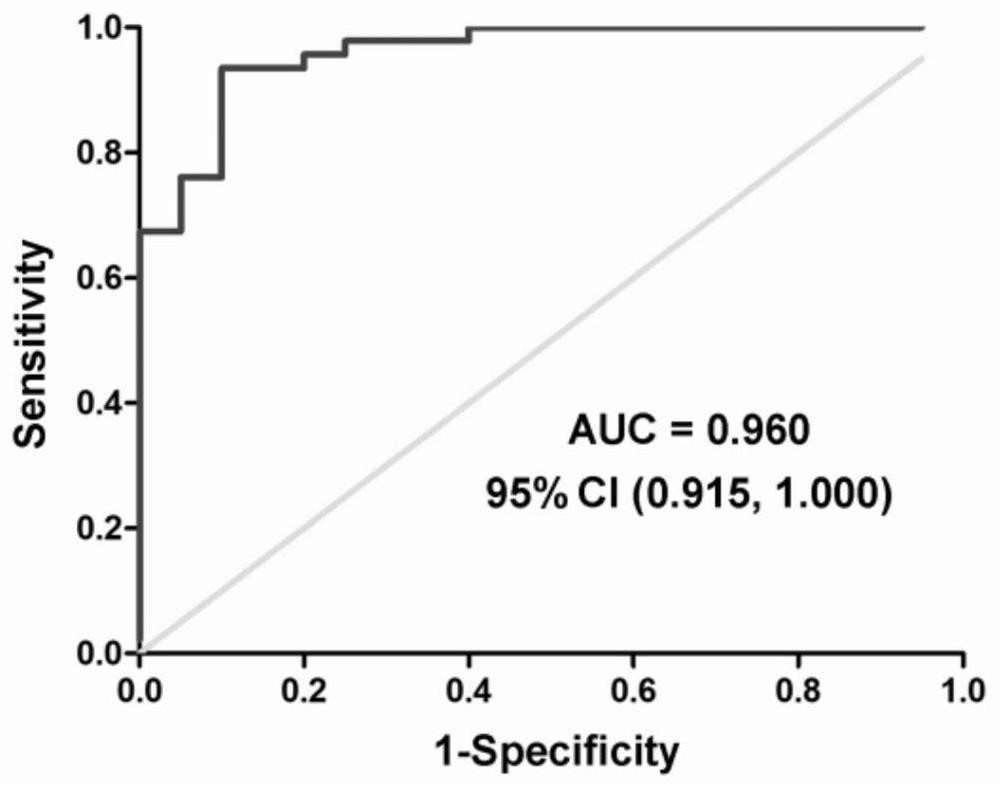
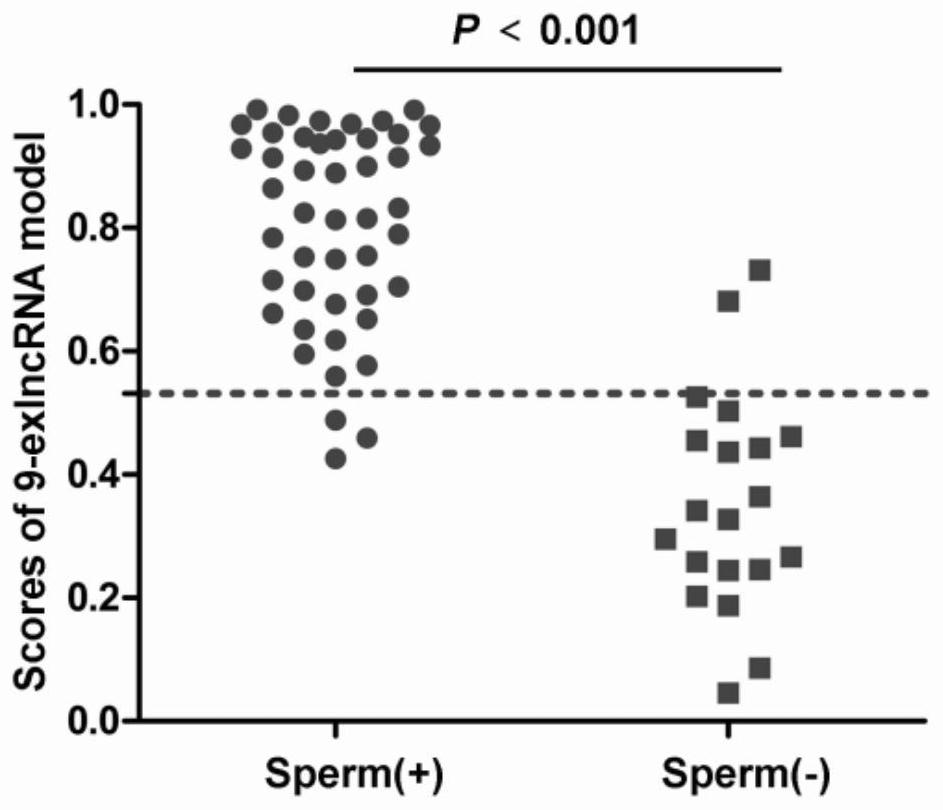
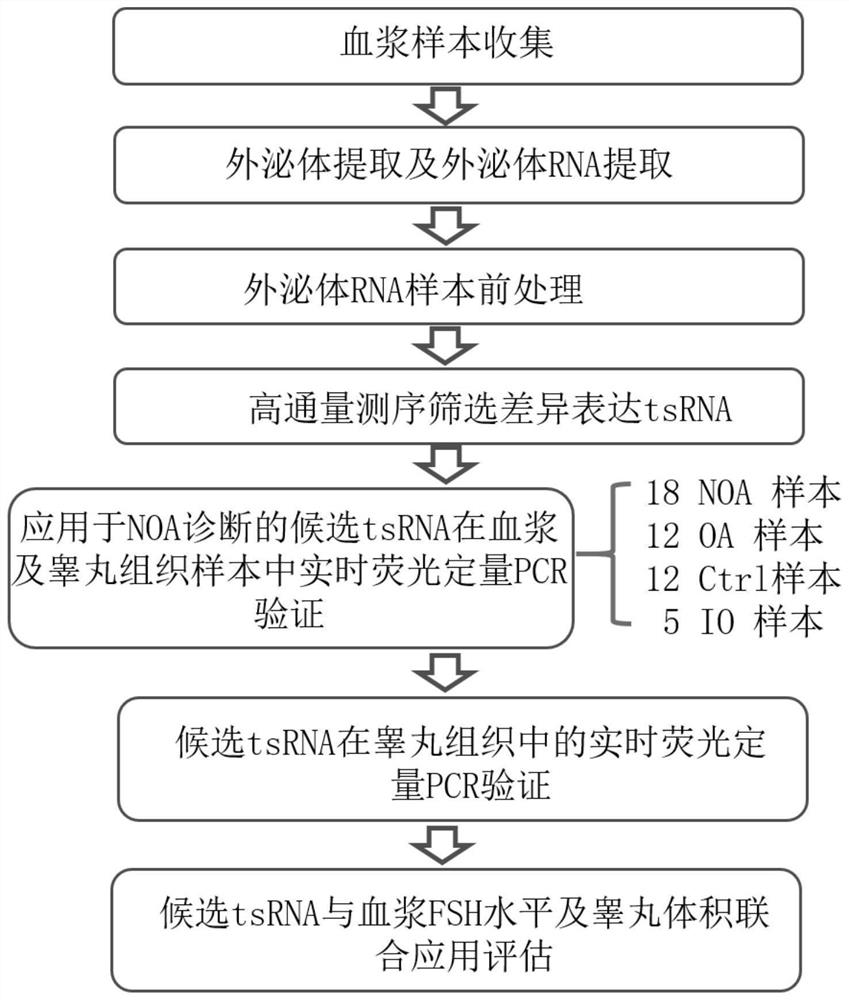
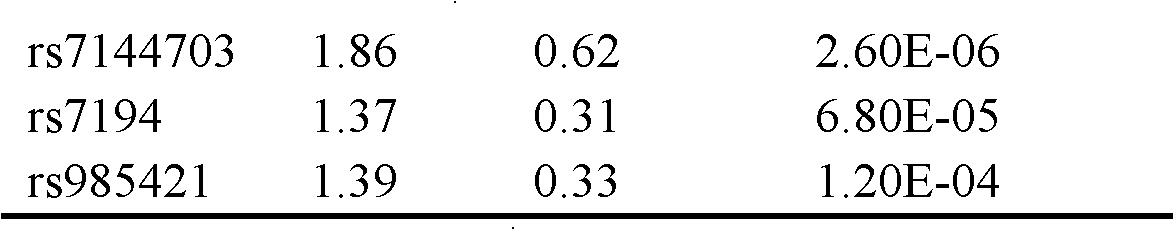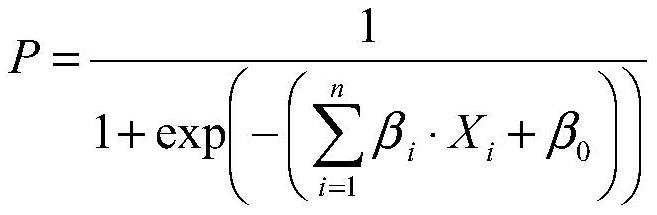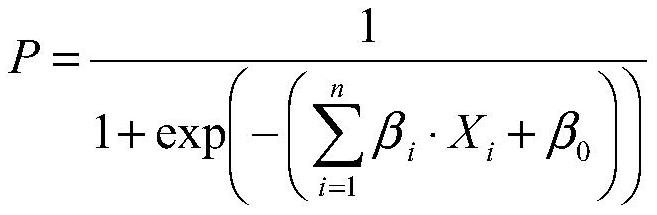Patents
Literature
38 results about "Non obstructive azoospermia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Non-obstructive azoospermia refers to no sperm in the semen because of abnormal sperm production. Sperm production problems can result from hormonal problems, testicular failure, or varicocele, or varicose veins in the testicles. Hormones are necessary for the testicles to produce sperm.
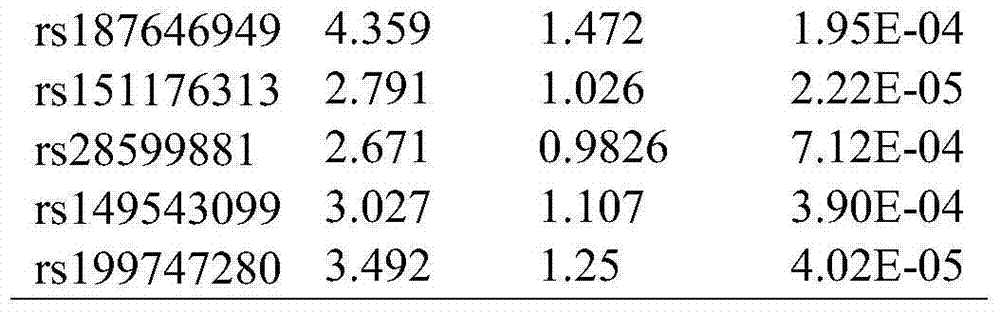
Single nucleotide polymorphism (SNP) marker related with clinically cryptogenic non-obstructive azoospermia aided diagnosis and application of SNP marker
ActiveCN102399898AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationGene engineeringReproductive medicine
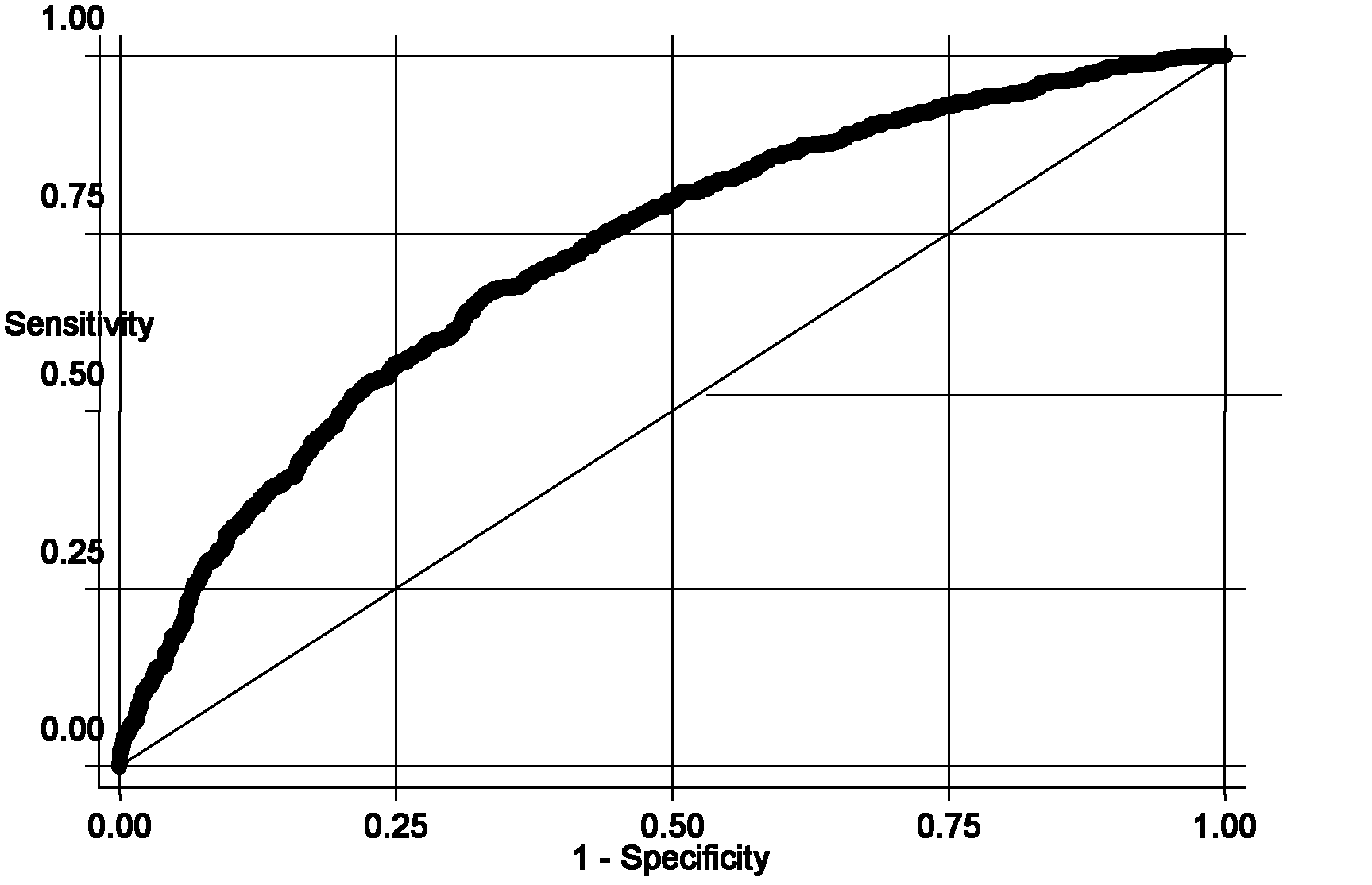
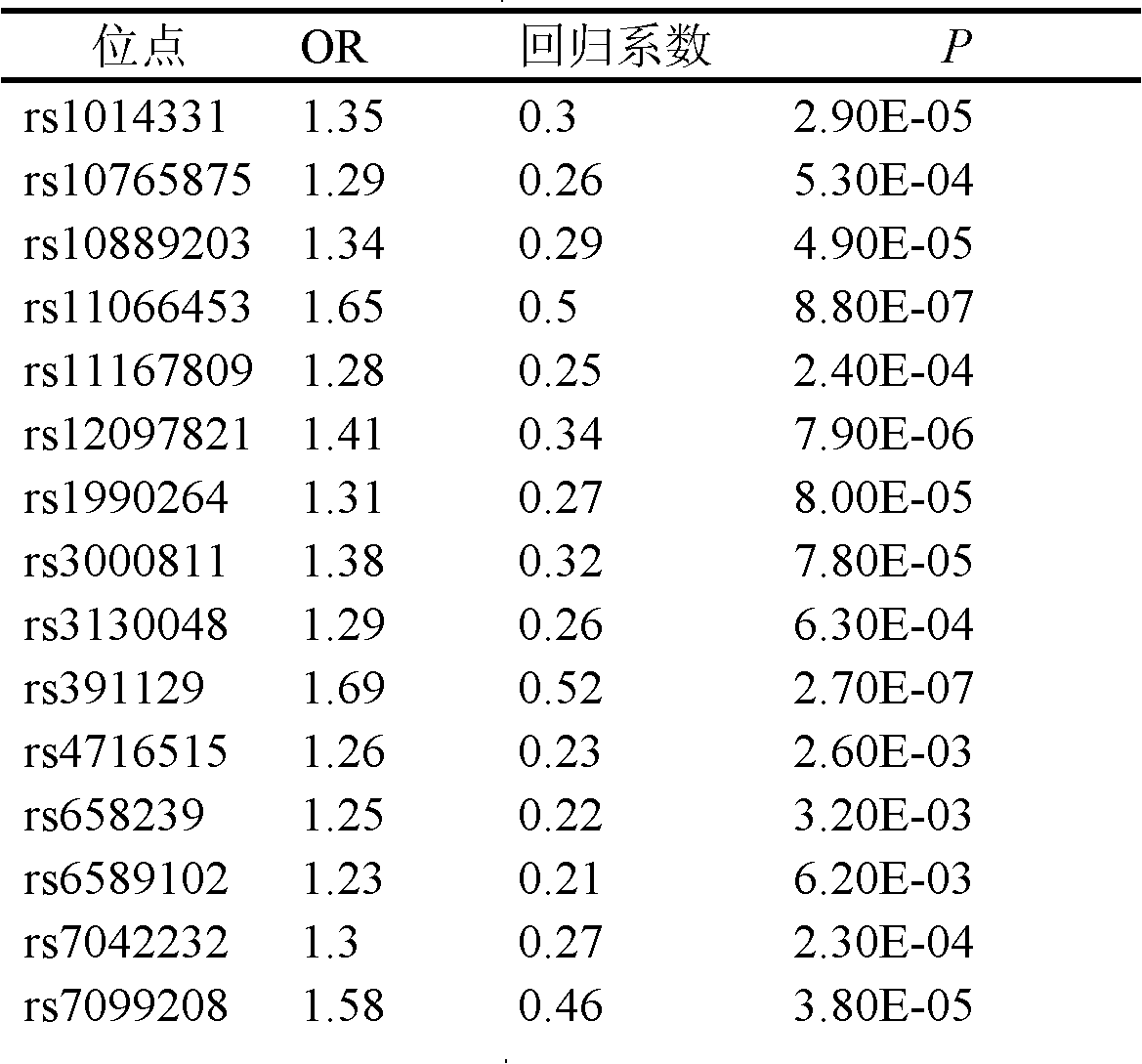
The invention belongs to the fields of gene engineering and reproductive medicine, and discloses a single nucleotide polymorphism (SNP) marker related with clinically cryptogenic non-obstructive azoospermia aided diagnosis and application of the SNP marker. The marker is a combination of rs1014331, rs10765875, rs10889203, rs11066453, rs11167809, rs12097821, rs1990264, rs3000811, rs3130048, rs391129, rs4716515, rs658239, rs6589102, rs7042232, rs7099208, rs7144703, rs7194 and rs985421. The marker can be used for preparing a clinically cryptogenic non-obstructive azoospermia aided diagnosis kit.
Owner:NANJING MEDICAL UNIV
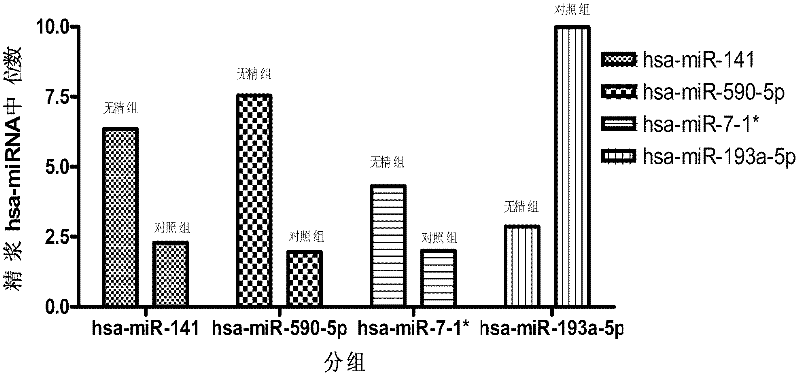
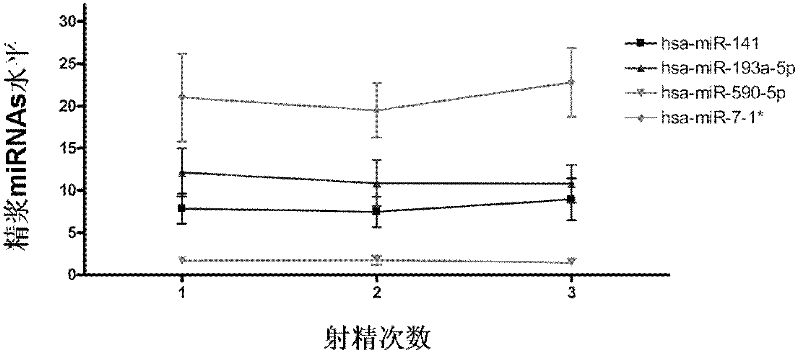
Seminal plasma microRNA markers associated with human non-obstructive azoospermia and their application
ActiveCN102296112AHigh sensitivityEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationDynamic monitoringGenetic engineering
The invention which belongs to the medical field of genetic engineering and reproduction discloses a seminal plasma miRNA marker associated with human non-obstructive azoospermia and an application thereof. The maker is selected from several of hsa-miR-141, hsa-miR-193a-5p, hsa-miR-590-5p and hsa-miR-7-1*. The maker which has specificities and sensitivities to the non-obstructive azoospermia can be used for the preparation of a reagent for non-obstructive azoospermia diagnosis or monitoring, so invasive diagnosis is avoided, repeated detection is realized, and the dynamic monitoring of the obstacle degree of sperm generation can be easily achieved.
Owner:NANJING MEDICAL UNIV
Reagent kit for detecting obstruction performance and non-obstruction performance non-spermatozoa symptom based on Eppin antibody
The invention relates to a reagent kit for detecting obstructive and non-obstructive azoospermia based on an Eppin antibody. The reagent kit comprises bovine serum albumin, a phosphate buffer solution, a TBST eluant, a rabbit anti-human Eppin antibody, a goat anti-rabbit IgG antibody, a chromogenic agent, a microfilter and a pyroxylin membrane arranged in the microfilter; in testing, seminal plasma which is to be tested is diluted through the phosphate buffer solution, is adsorbed and fixed on the pyroxylin membrane of the microfilter and is closed through the bovine serum albumin; and the pyroxylin membrane closed by the albumen is subjected to first washing through the TBST eluant, is incubated through the rabbit anti-human Eppin antibody, is subjected to secondary washing through TBST, is incubated through the goat anti-rabbit IgG antibody and is subjected to chromogenic discrimination through the chromogenic agent. The reagent kit has the advantages that the reagent kit has no wound, can alleviate the pain of a patient receiving seminal duct radiography and testicle biopsy, can save mass inspection expense needed by the prior seminal duct radiography and relevant inspection, has high sensitivity, can reach the molecular level, is simple, feasible and rapid and has important application prospect and practical value.
Owner:JIANGSU PROVINCE HOSPITAL
A clinically unexplained NOA-related mitochondrial DNA SNP marker and applications thereof
ActiveCN103290006AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationDiseaseTrue positive rate
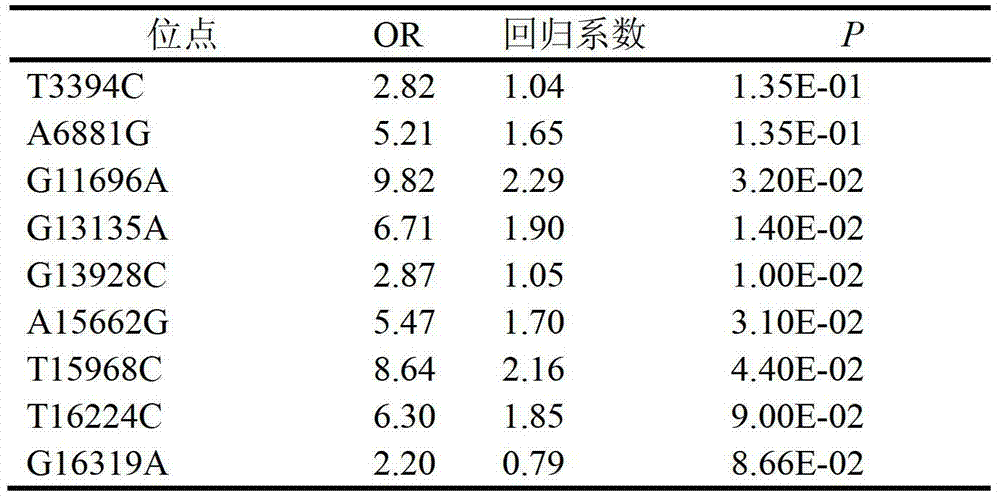
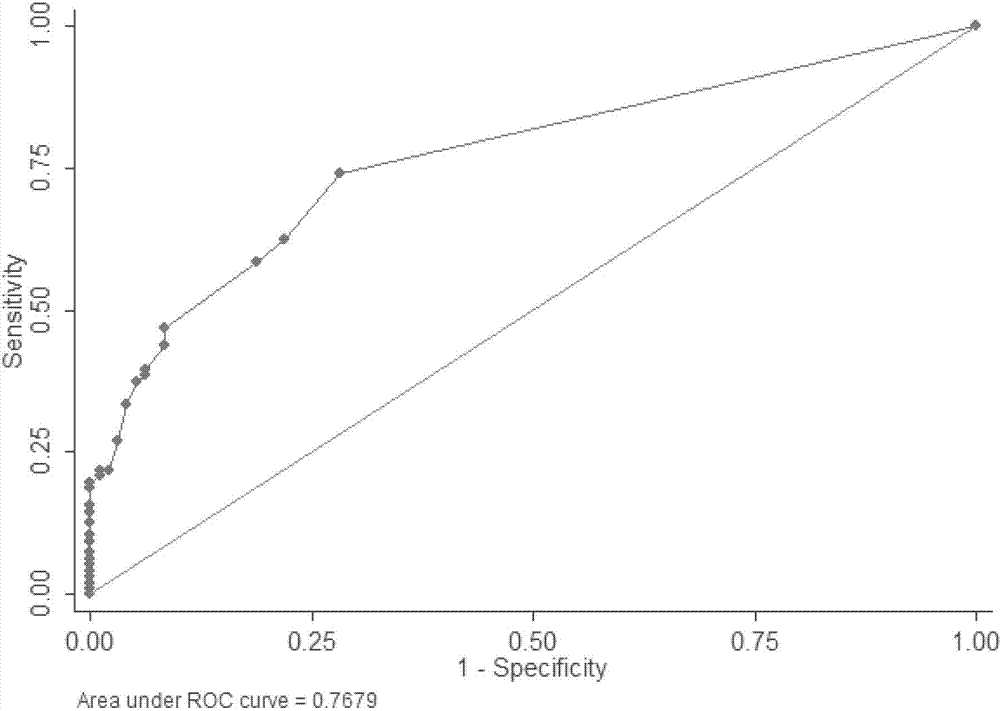
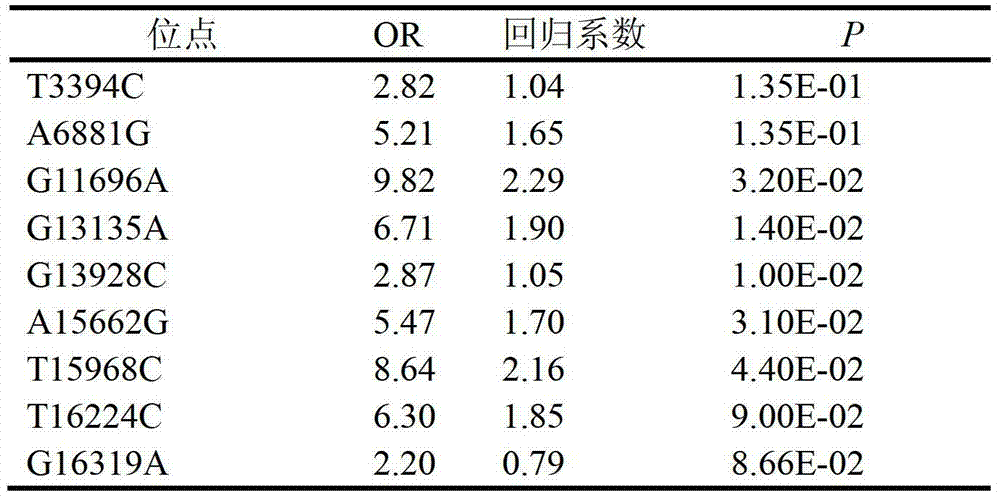
The invention relates to a clinically unexplained NOA (non-obstructive azoospermia)-related mitochondrial DNA SNP (single nucleotide polymorphism) marker and applications thereof, belonging to the fields of genetic engineering and reproductive medicine. The marker is a combination of the following mitochondrial DNA SNP sites: T3394C, A6881G, G11696A, G13135A, G13928C, A15662G, T15968C, T16224C and G16319A. The marker and specific primers and / or specific probes thereof can be used for auxiliary diagnosis of the clinically unexplained NOA, greatly improving the sensitivity and specificity of diagnosis.
Owner:夏彦恺
Seminal plasma exosome tsRNA markers related to non-obstructive azoospermia diagnosis and application of seminal plasma exosome tsRNA markers
ActiveCN112251508AEasy diagnosisImprove discriminationMicrobiological testing/measurementDNA/RNA fragmentationTesticular volumeBlood plasma
The invention discloses seminal plasma exosome tsRNA markers related to non-obstructive azoospermia diagnosis and application of the seminal plasma exosome tsRNA markers. The markers are tRF-Pro-AGG-003 and tRF-Val-AAC-010, the sequence of tRF-Pro-AGG-003 is SEQ ID NO.1, and the sequence of tRF-Val-AAC-010 is SEQ ID NO.2. The markers have specificity and sensitivity on non-obstructive azoospermiaand can be used for preparing a diagnostic kit for non-obstructive azoospermia. The two seminal plasma exosome markers tRF-Pro-AGG-003 and tRF-Val-AAC-010 are differentially expressed in seminal plasma of patients suffering from non-obstructive azoospermia and obstructive azoospermia, non-obstructive azoospermia can be well diagnosed, an azoospermia sperm taking result can be predicted, and the effect of the markers is superior to the indexes such as the common plasma follicle stimulating hormone level and the testicular volume.
Owner:江苏阔然生物医药科技有限公司
Rapid typing detection kit for ejaculated sperm cells and rapid typing detection method for ejaculated sperm cells
InactiveCN110286080AChange dependenciesLow application costIndividual particle analysisBiological testingGlycerolBovine serum albumin
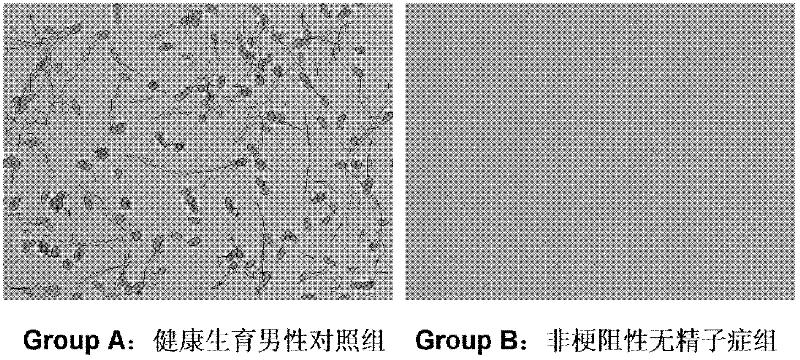
The invention discloses a rapid typing detection kit for ejaculated sperm cells. The kit comprises a PBS solution, a calcium-free HBSS buffer solution, a fixing solution, a staining reagent and a quality control product. The PBS solution and the calcium-free HBSS buffer solution both comprise one or more of bovine serum albumin, fetal bovine serum, glycerol serum, protein and glycerol; the staining reagent comprises a cell permeabilizing agent and a fluorescently labeled antibody. The invention also provides a rapid typing detection method for ejaculated sperm cells by adopting the kit mentioned above. The rapid typing detection kit for ejaculated sperm cells and the rapid typing detection method for ejaculated sperm cells can be used for quickly determining the types and the quantity of spermatogenic cells in the ejaculated sperm cells, further determining whether the ejaculated sperm cells are obstructive or non-obstructive azoospermia, solve the problem that clinical evaluation aiming at the current semen cytology in China lacks a quick and accurate detection reagent in the current clinic, provide guidance for a clinician to select a treatment scheme, and practically solve the problem of a patient.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Reagent kit for predicting semen collection outcome of patient suffering from non-obstructive azoospermia
ActiveCN111944895AAchieve three-stage amplificationMicrobiological testing/measurementHybridisationPhysiologySpermatid
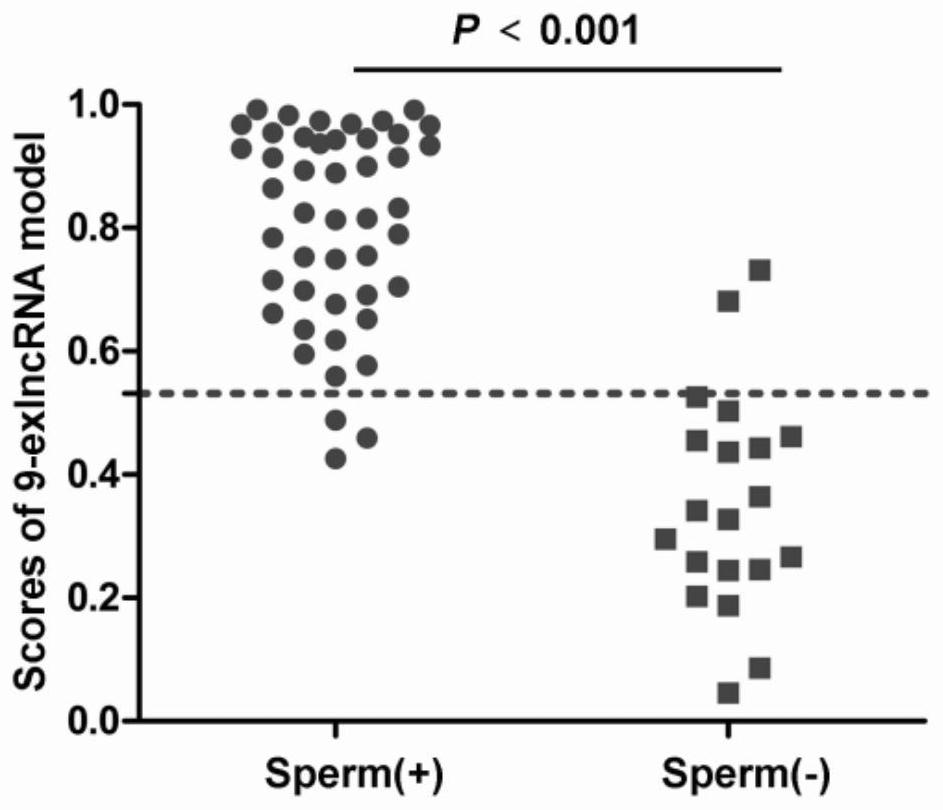
The invention relates to the field of biological medicine, in particular to a reagent kit for predicting semen collection outcome of a patient suffering from non-obstructive azoospermia. The reagent kit comprises a reagent for detecting the expression level of lncRNA in a sample, and the lncRNA comprises at least one of SPATA42, CCDC37-DT, GABRG3-AS1, LOC440934, LOC100505685, LOC101929088 (XR_927561.2), LOC101929088 (XR_001745218.1), LINC00343 and LINC00301. According to the reagent kit, the sperm extraction outcome of the patient suffering from NOA can be noninvasively and accurately predicted before an operation, and the reagent kit has sufficient scientificity and practicability.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
Plasma exosome tsRNA marker related to non-obstructive azoospermia diagnosis and application thereof
ActiveCN112176052APrediction of sperm retrieval resultsEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationTesticular volumeBlood plasma
The invention discloses a plasma exosome tsRNA marker related to non-obstructive azoospermia diagnosis and application thereof. The marker is tRF-Gly-GCC-002, wherein the sequence of the tRF-Gly-GCC-002 is shown as SEQ ID NO.1. The marker has specificity and sensitivity to non-obstructive azoospermia, and can be used for preparing a diagnostic kit for non-obstructive azoospermia; and the plasma exosome marker tRF-Gly-GCC-002 is differentially expressed in plasma of patients with non-obstructive azoospermia and obstructive azoospermia, the plasma exosome marker tRF-Gly-GCC-002 can well diagnosethe non-obstructive azoospermia, and can predict the azoospermia sperm taking result, and the effect of the plasma exosome marker tRF-Gly-GCC-002 is superior to common indexes such as the plasma follicle stimulating hormone level and the testicular volume.
Owner:XUZHOU MEDICAL UNIV
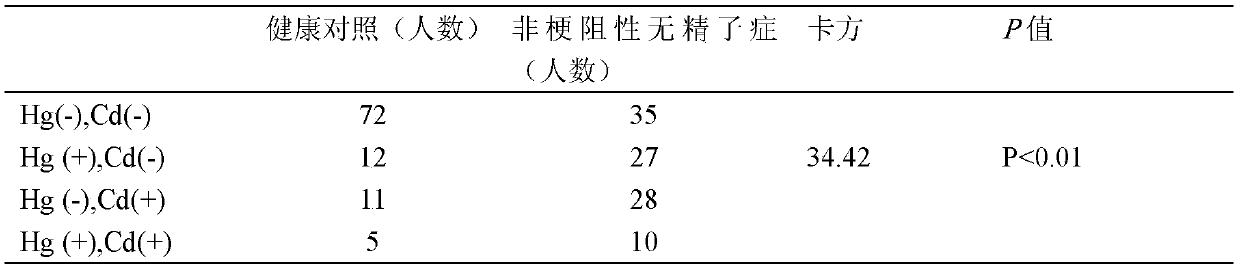
Application of mercury and cadmium mixed exposure detection in auxiliary diagnosis of non-obstructive azoospermia
PendingCN109254070AIncreased sensitivityImprove featuresMaterial analysis by electric/magnetic meansMixed exposureMedicine
The invention belongs to the field of analytical chemistry and clinical medicine, and discloses application of mercury and cadmium mixed exposure detection in auxiliary diagnosis of non-obstructive azoospermia. An exposed biomarker related to the non-obstructive azoospermia is mercury and cadmium mixed exposure. The concentration of mercury and cadmium in whole blood is detected by ICP-MS detection, the application can be used for auxiliary diagnosis of the non-obstructive azoospermia, a few amount of blood is used, and the application is simple, rapid and accurate to operate and has relatively good clinical promotion value.
Owner:NANJING MEDICAL UNIV
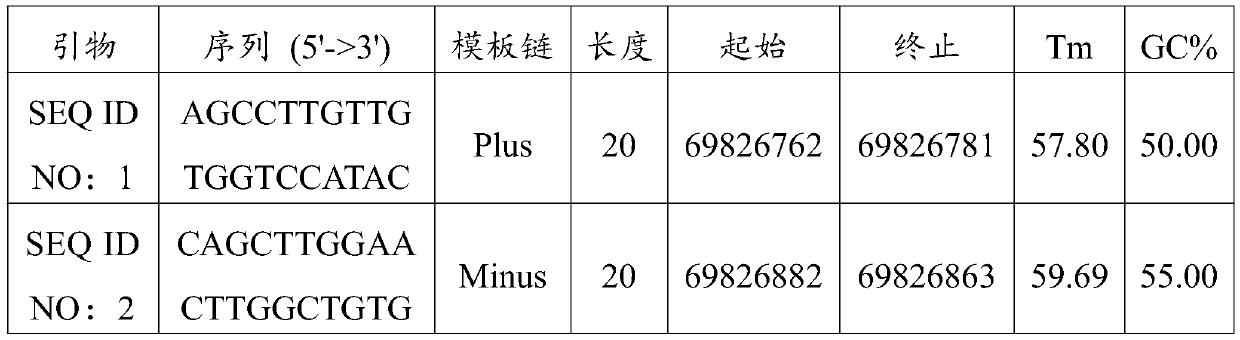
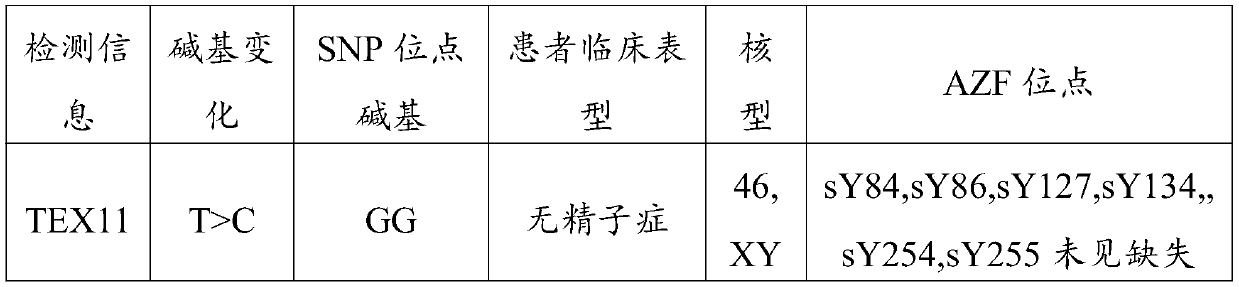
Non-obstructive azoospermia auxiliary diagnosis gene detection kit
The invention discloses a non-obstructive azoospermia auxiliary diagnosis gene detection kit, relates to the field of azoospermia diagnosis, and solves the problem of difficult clinical diagnosis of male infertility. The non-obstructive azoospermia auxiliary diagnosis gene detection kit comprises a specific PCR primer, a PCR buffer solution, dNTPs and DNA polymerase, wherein the specific PCR primer is a group of specific primers for detecting the 69826819 mutation site in the non-obstructive azoospermia TEX11 gene. A specific primer SEQ ID NO: 1 and a specific primer SEQ ID NO: 2 are designed,the specific primers have higher specificity, the non-obstructive azoospermia auxiliary diagnosis gene detection kit consisting of the specific primers is adopted to detect the 69826819 mutation sitein the TEX11 gene of a male patient, and the detection rate of the gene mutation is 100%.
Owner:YINFENG JILIN BIOLOGICAL ENG TECH CO LTD +2
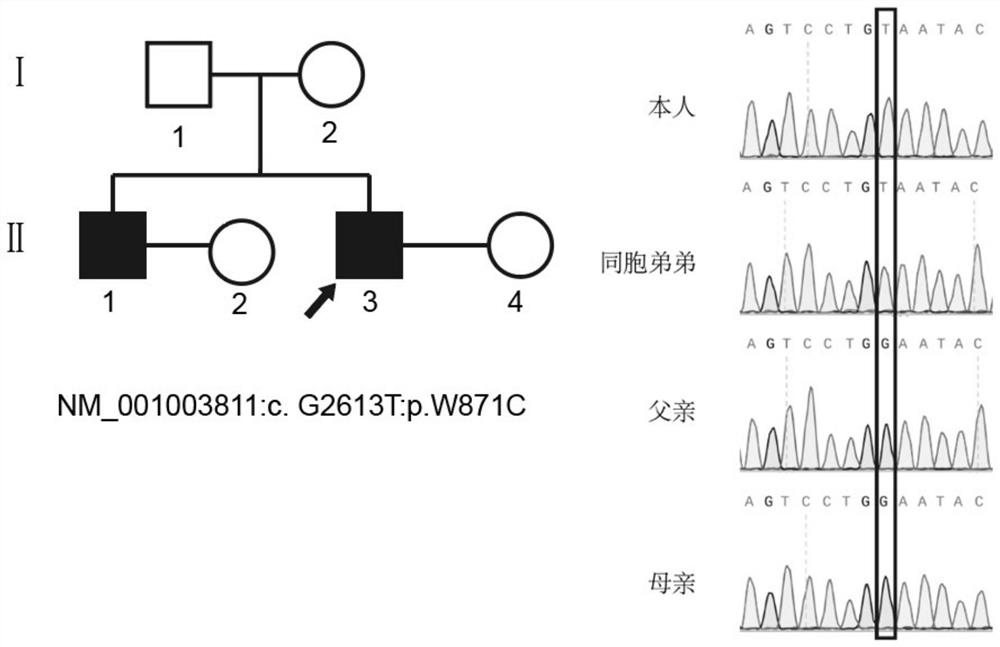
Application of TEX11 gene pathogenic mutation in preparation of diagnostic kit for detecting non-obstructive azoospermia
ActiveCN113308531AReduce financial burdenQuick checkMicrobiological testing/measurementAgainst vector-borne diseasesGenes mutationGene screening
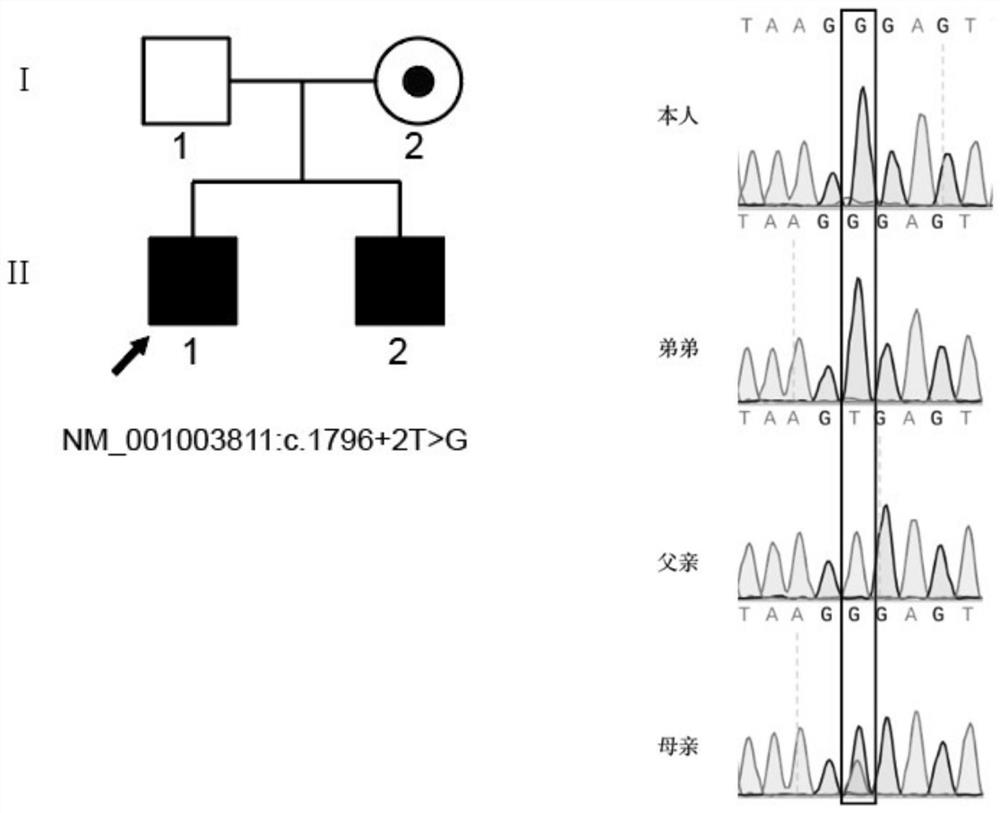
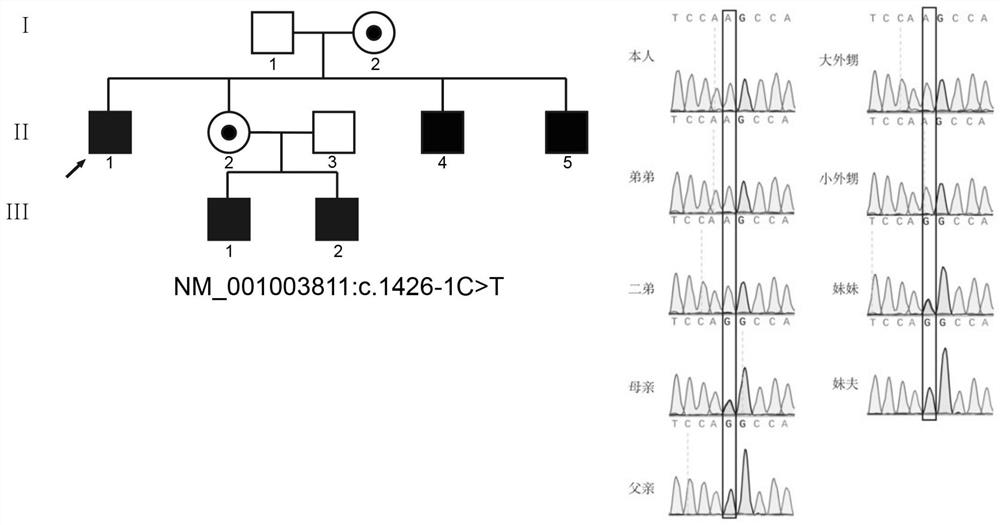
The invention relates to the technical field of biology, in particular to application of TEX11 gene pathogenic mutation in preparation of a diagnostic kit for detecting non-obstructive azoospermia. According to the method, peripheral blood DNA (deoxyribonucleic acid) is firstly extracted, then a target gene is subjected to PCR (polymerase chain reaction) amplification, and finally, the pathogenic mutation of the TEX11 gene is finally verified in four families: family 1: NM_001003811:c.G2613T:p.W871C, family 2: NM_001003811:c.1426-1C>T; family 3: NM_001003811:c.1796+2T>G; and family 4: NM_001003811:c.857delA:p.K286R fs*5. The method has the advantages that an effective way is provided for non-obstructive azoospermia gene diagnosis, prenatal gene screening and genetic counseling.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Serum miRNA combination as molecular marker for assessing non-obstructive azoospermia
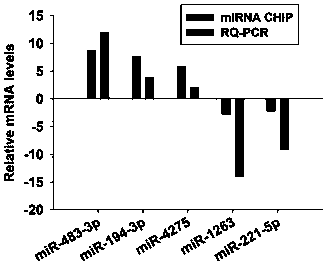
InactiveCN110592204AEasy to operateImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationSerum igeSerum mirna
The invention discloses an application of a serum miRNA combination as molecular marker for assessing non-obstructive azoospermia. The serum miRNA combination includes hsa-miR-1263, hsa-miR-221-5p, hsa-miR-483-3p, hsa-miR-4275 and hsa-miR-194-3p. Through the miRNA combination, a reagent kit for assessing the non-obstructive azoospermia is prepared. The serum miRNA for assessing the non-obstructiveazoospermia is obtained, further a detection reagent kit containing primers used for quantitative RQ-PCR detection of the serum miRNA combination is obtained, detection is performed through the detection primers and the detection reagent kit, the operation is simple, the accuracy is high, and the serum miRNA combination is worth of extensive popularization.
Owner:广东省计划生育科学技术研究所
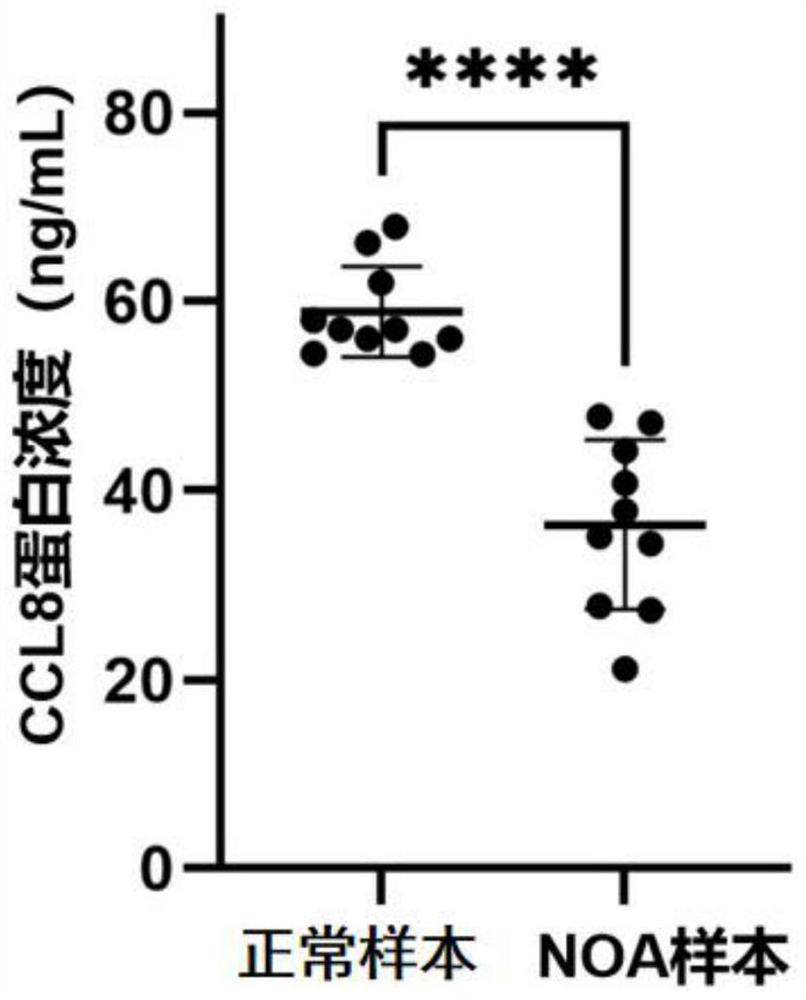
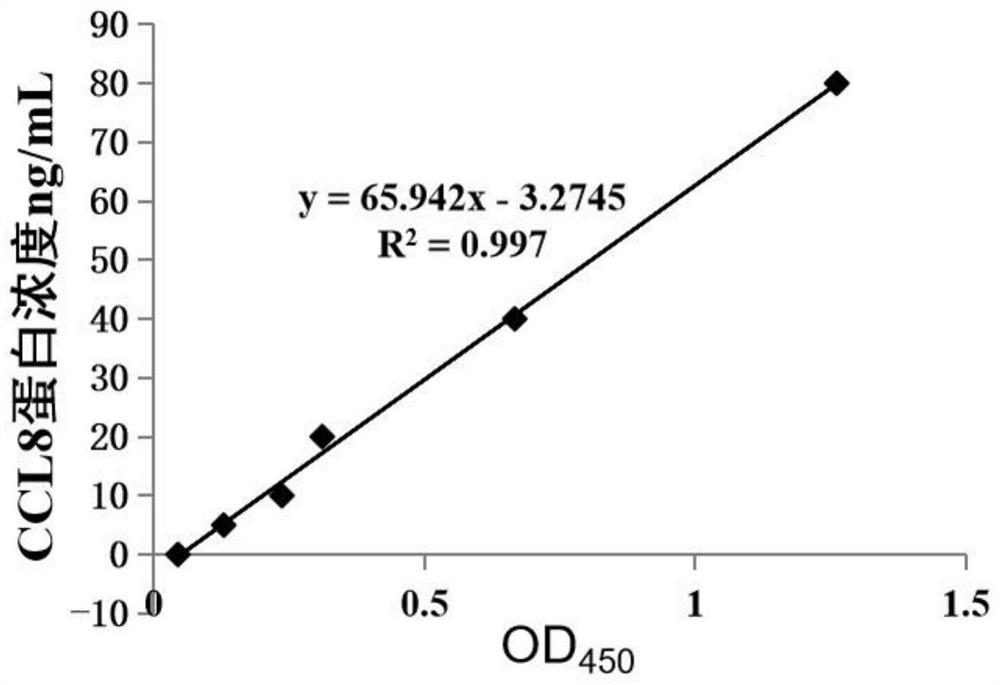
CCL8 protein as biomarker of non-obstructive azoospermia and application of CCL8 protein
The invention discloses a CCL8 protein serving as a biomarker of non-obstructive azoospermia and application of the CCL8 protein. The biomarker comprises a CCL8 protein. The invention finds that the concentration of the CCL8 protein in the seminal fluid of the NOA patient is obviously lower than that of a normal male for the first time, and proposes that the CCL8 protein can be used as a potential early warning molecule for evaluating the spermatogenic dysfunction of the NOA patient, so that expansion and diagnosis of NOA indexes are facilitated, the diagnosis range is expanded, and the CCL8 protein has important significance in the field of NOA diagnosis.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV
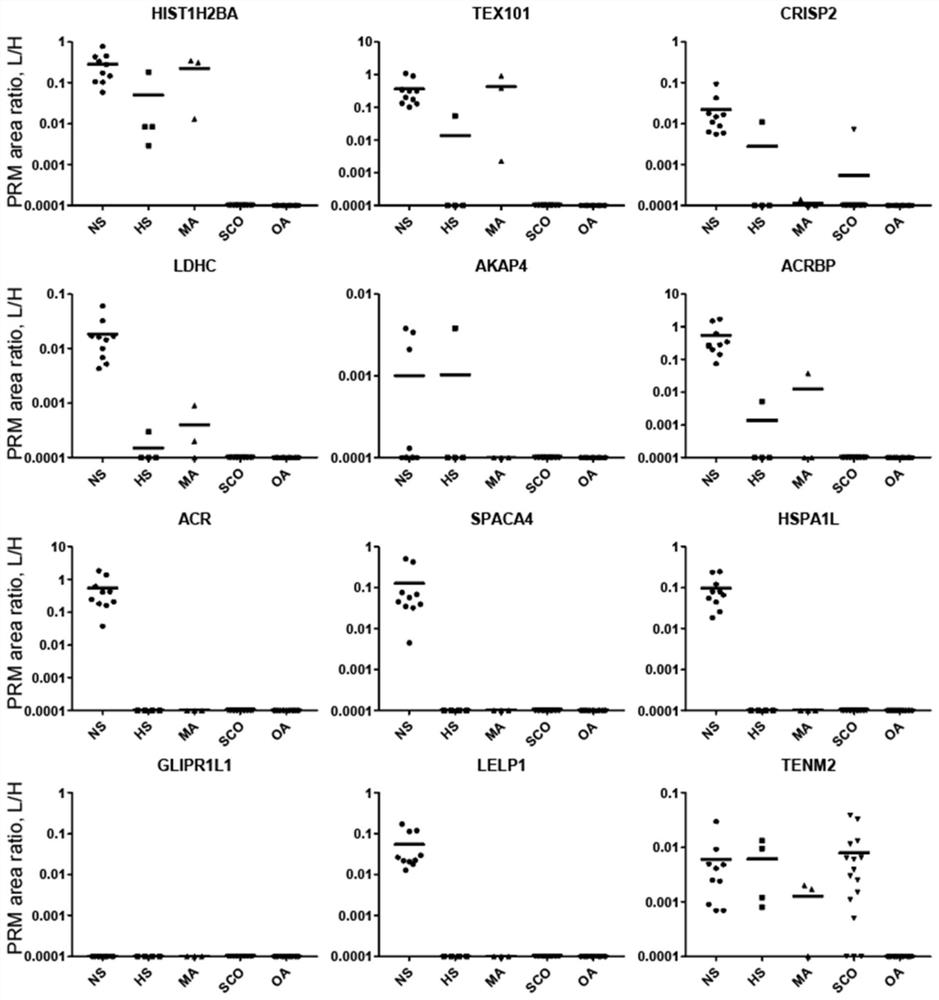
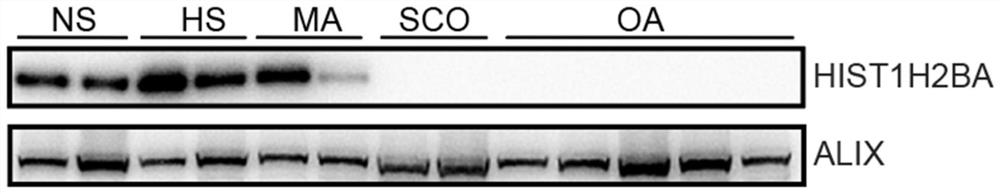
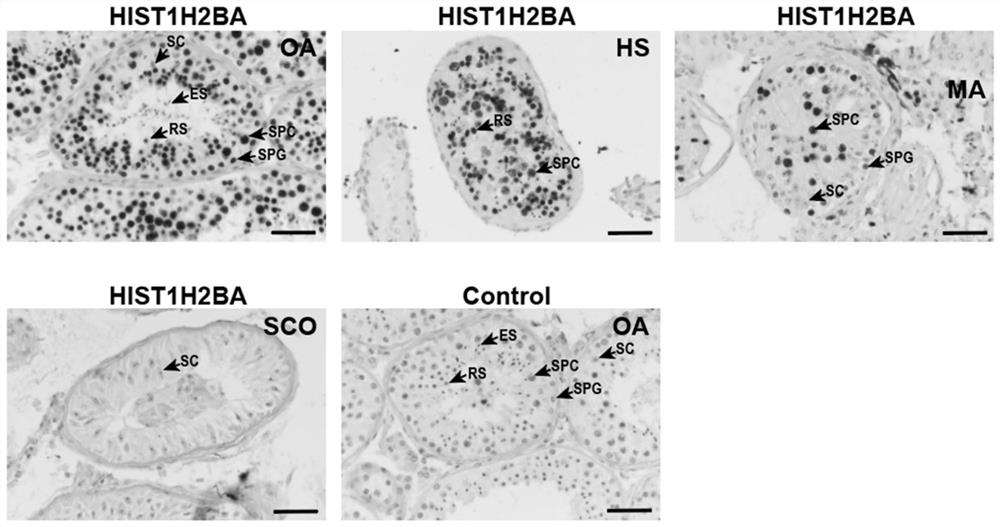
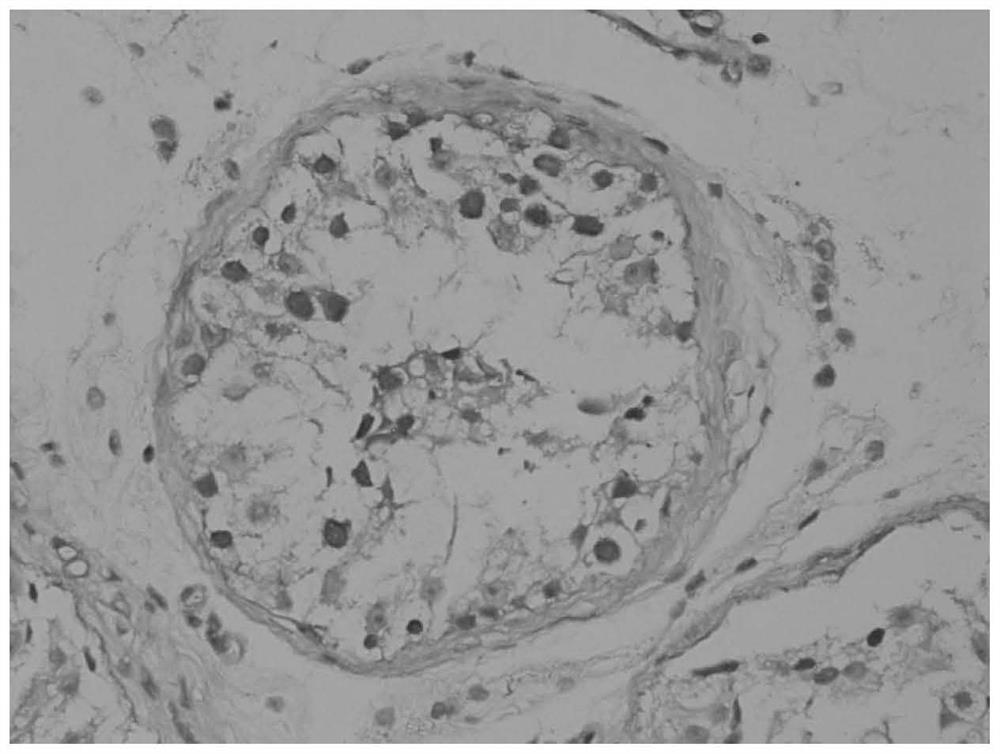
Application of seminal plasma extracellular vesicle HIST1H2BA protein
The invention discloses application of seminal plasma extracellular vesicle HIST1H2BA protein, belongs to the field of protein detection, and particularly relates to application of the seminal plasma extracellular vesicle HIST1H2BA protein in biomarkers of seminal plasma extracellular vesicles of different pathological types of non-obstructive azoospermia. The HIST1H2BA provided by the invention can be used for distinguishing NOA-SCO from other pathological types and predicting the spermatogenesis condition in testis.
Owner:NANJING MEDICAL UNIV
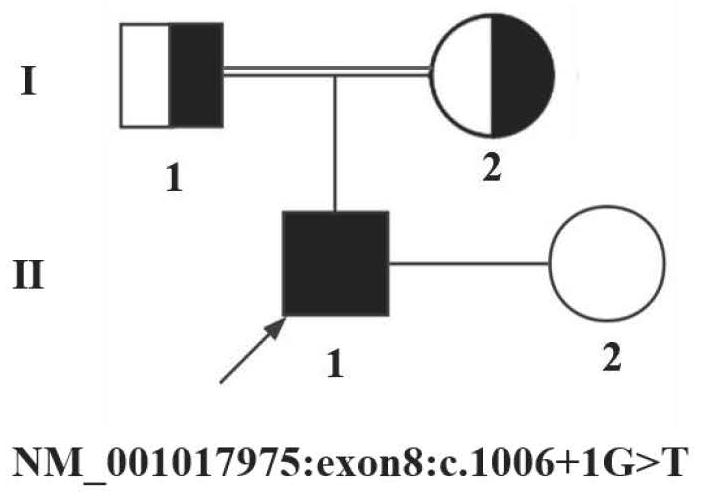
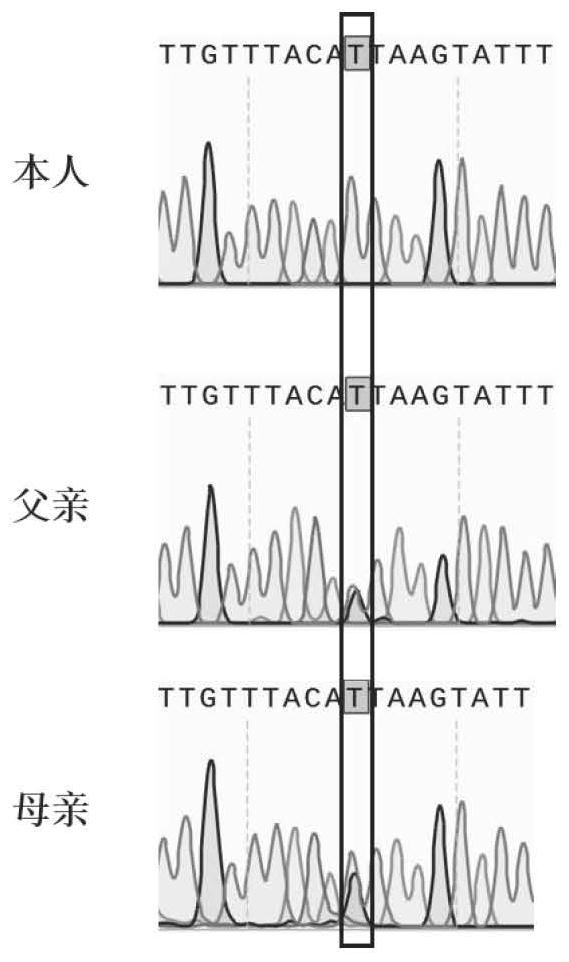
Application of HFM1 gene in preparation of diagnostic kit for detecting non-obstructive azoospermia
ActiveCN114438194AEfficient detectionReduce financial burdenMicrobiological testing/measurementDNA/RNA fragmentationNucleotideGene screening
The invention relates to a non-obstructive azoospermia detection kit, the kit comprises a primer pair for detecting a genetic marker related to non-obstructive azoospermia, the nucleotide sequence of the genetic marker is the sequence of an HFM1 gene, and the mutation site of the sequence is c.1006 + 1Ggt; t. The invention also provides application of the detection kit in diagnosis of non-obstructive azoospermia and application of the HFM1 gene in preparation of the diagnostic kit for detecting non-obstructive azoospermia. The invention provides a new application of the HFM1 gene, so that an effective way for performing non-obstructive azoospermia gene diagnosis, prenatal gene screening and genetic counseling is provided; the application effect shows that the SNP site of the gene and the detection primer provided by the invention can be effectively used for rapid detection of the HFM1 gene mutation site in clinical patients and fetal villus or amniotic fluid.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
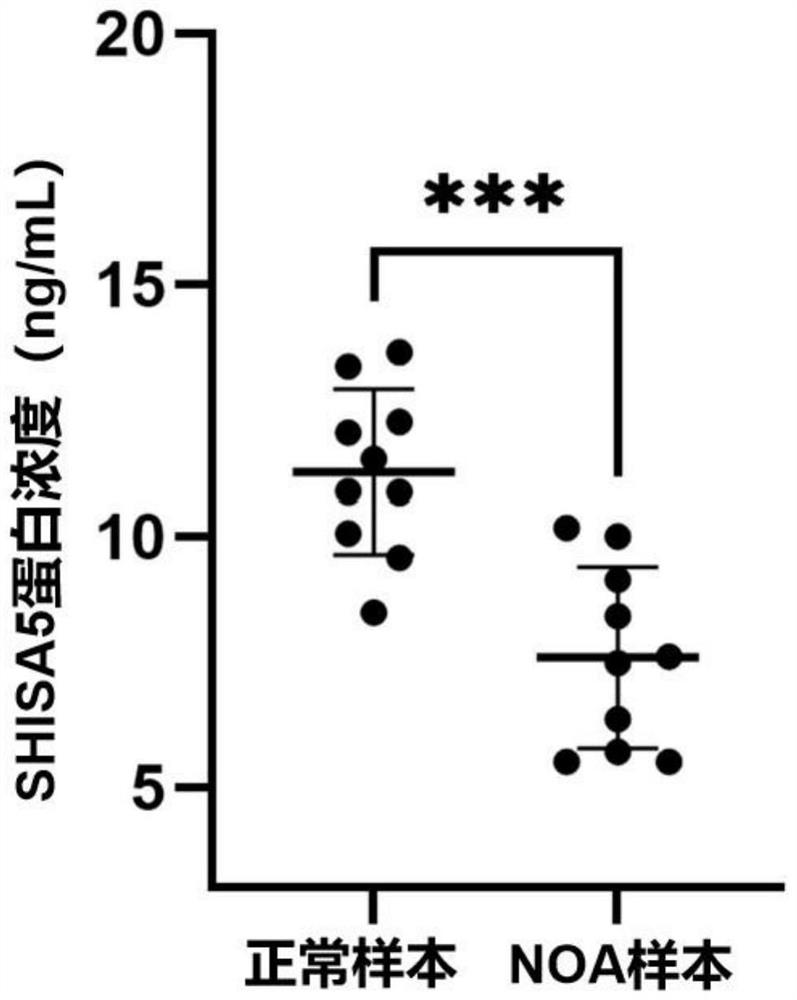
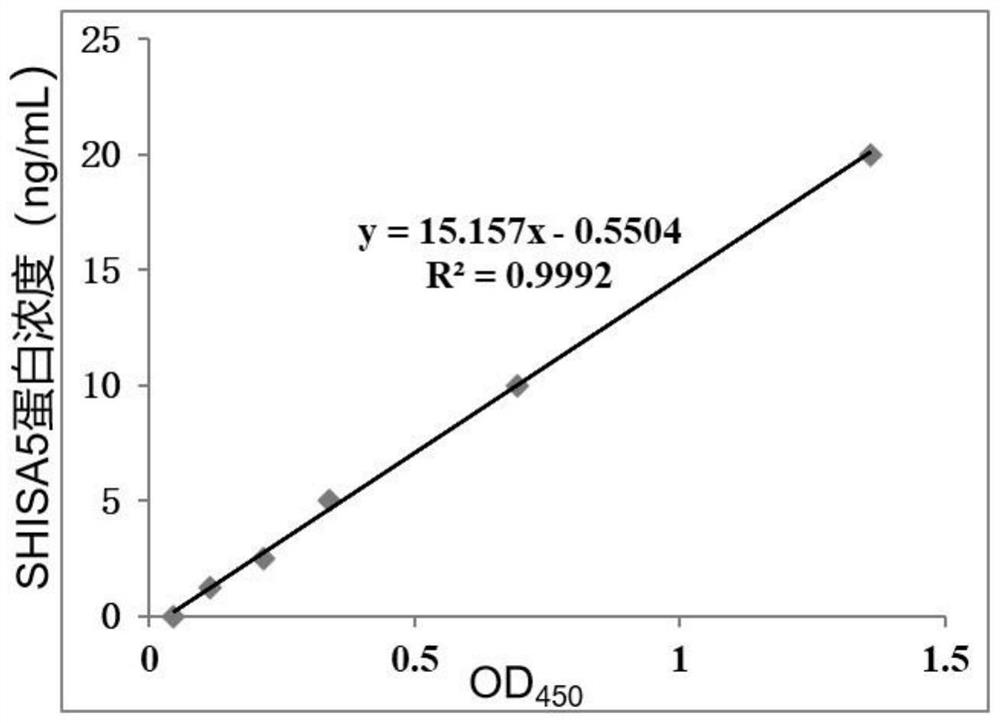
Biomarker related to non-obstructive azoospermia and application thereof
The invention discloses a biomarker related to non-obstructive azoospermia and an application of the biomarker. The biomarker comprises an SHISA5 protein. According to the present invention, the concentration of the Wnt receptor inhibition protein SHISA5 in the NOA patient semen is significantly lower than the concentration of the Wnt receptor inhibition protein SHISA5 in the normal male semen for the first time, and the SHISA5 protein can be adopted as the potential early warning molecule for evaluating the spermatogenic dysfunction of the NOA patient, such that the NOA diagnosis index can be easily expanded so as to expand the diagnosis range, and the important significance is provided for the NOA diagnosis field.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV
Plasma exosome miRNA markers related to diagnosis of primary non-obstructive azoospermia and application thereof
ActiveCN112195232APrediction of sperm retrieval resultsEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationAzoospermiaTesticle
The invention discloses plasma exosome miRNA markers related to diagnosis of primary non-obstructive azoospermia and application thereof. The markers are hsa-miR-513c-5p and hsa-miR-202-5p, the sequence of the hsa-miR-513c-5p is shown as SEQ ID NO.1, and the sequence of the hsa-miR-202-5p is shown as SEQ ID NO.2. The markers have specificity and sensitivity to the non-obstructive azoospermia, andcan be used to prepare a diagnostic kit for the non-obstructive azoospermia. The two plasma exosome markers hsa-miR-513c-5p and hsa-miR-202-5p disclosed by the invention are differentially expressed in the plasma of patients with non-obstructive azoospermia and obstructive azoospermia, so that the non-obstructive azoospermia can be diagnosed better, the sperm taking result of azoospermia can be predicted, and the effect is superior to common indexes such as plasma follicle-stimulating hormone level and testis volume.
Owner:XUZHOU MEDICAL UNIV
A plasma exosomal miRNA marker associated with the diagnosis of primary non-obstructive azoospermia and its application
ActiveCN112195232BPrediction of sperm retrieval resultsEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationTesticular volumeDisease
The invention discloses a plasma exosome miRNA marker related to the diagnosis of primary non-obstructive azoospermia and its application. The markers are hsa-miR-513c-5p and hsa-miR-202-5p, the sequence of hsa-miR-513c-5p is SEQ ID NO.1, and the sequence of hsa-miR-202-5p is SEQ ID NO. 2. The marker has specificity and sensitivity to non-obstructive azoospermia, and can be used to prepare a diagnostic kit for non-obstructive azoospermia. The two plasma exosome markers hsa-miR-202-5p and hsa-miR-513c-5p of the present invention are differentially expressed in the plasma of patients with non-obstructive azoospermia and obstructive azoospermia, which can better It can diagnose non-obstructive azoospermia, and can predict the result of sperm retrieval in azoospermia, and its effect is better than commonly used indicators such as plasma follicle stimulating hormone level and testicular volume.
Owner:XUZHOU MEDICAL UNIV
A mitochondrial DNA SNP marker associated with noa of unknown clinical cause and its application
ActiveCN103290006BIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationDiseaseTrue positive rate
The invention relates to a clinically unexplained NOA (non-obstructive azoospermia)-related mitochondrial DNA SNP (single nucleotide polymorphism) marker and applications thereof, belonging to the fields of genetic engineering and reproductive medicine. The marker is a combination of the following mitochondrial DNA SNP sites: T3394C, A6881G, G11696A, G13135A, G13928C, A15662G, T15968C, T16224C and G16319A. The marker and specific primers and / or specific probes thereof can be used for auxiliary diagnosis of the clinically unexplained NOA, greatly improving the sensitivity and specificity of diagnosis.
Owner:夏彦恺
Low-frequency SNV (single nucleotide variant) marker related to assisted diagnosis of NOA (non-obstructive azoospermia) with unknown clinical reasons and application thereof
ActiveCN104263822AHigh sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationGene engineeringBiology
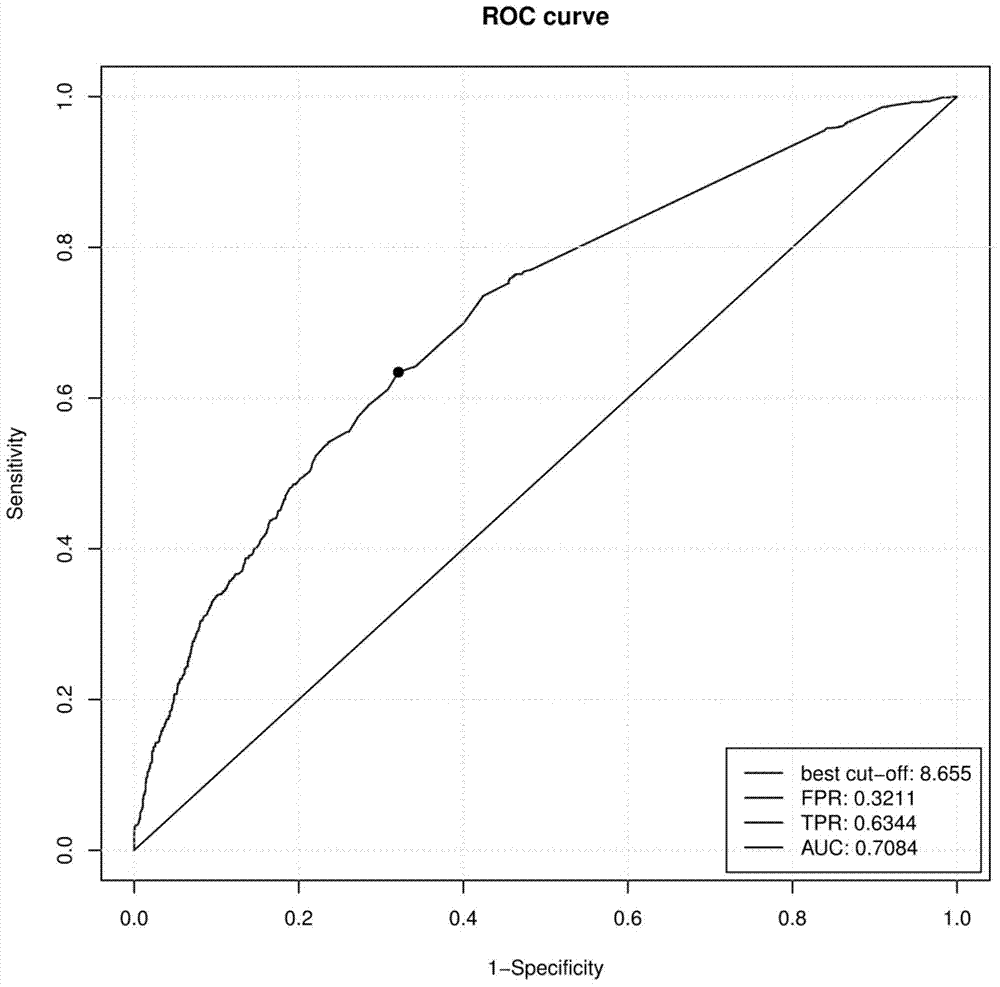
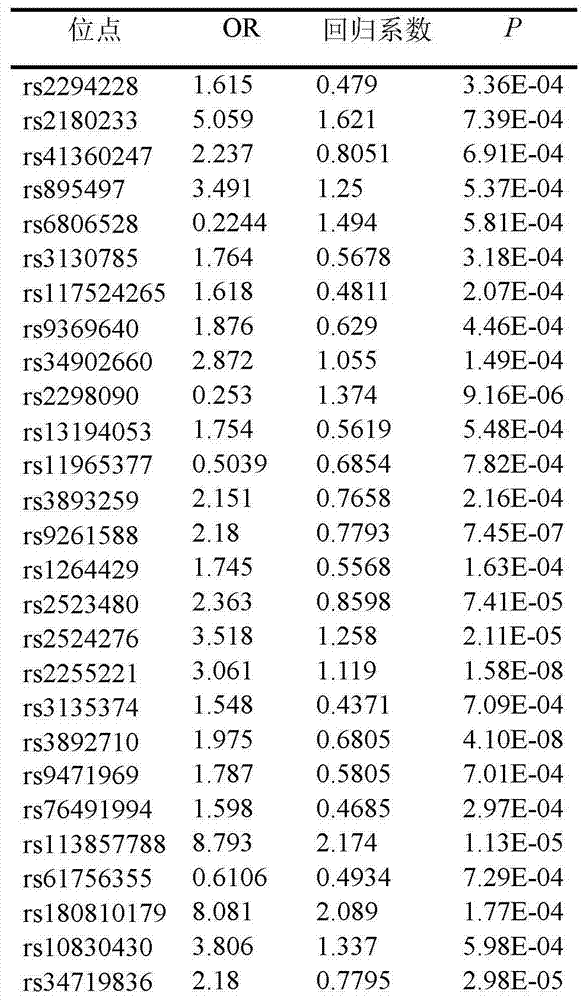
The invention belongs to the fields of gene engineering and reproductive medicine, and discloses a low-frequency SNV (single nucleotide variant) marker related to assisted diagnosis of NOA (non-obstructive azoospermia) with unknown clinical reasons and application thereof. The marker is a combination of 32 low-frequency SNVs, including rs2294228, rs2180233, rs41360247, rs895497, rs6806528, rs3130785, rs117524265, rs9369640, rs34902660, rs2298090, rs13194053, rs11965377, rs3893259, rs9261588, rs1264429, rs2523480, rs2524276, rs2255221 and the like. The marker can be used for preparing an assisted diagnosis kit for non-obstructive azoospermia with unknown clinical reasons.
Owner:NANJING MEDICAL UNIV
A kit for predicting sperm retrieval outcome in patients with non-obstructive azoospermia
ActiveCN111944895BAchieve three-stage amplificationMicrobiological testing/measurementHybridisationBiomedicineSpermatid
The invention relates to the field of biomedicine, in particular to a kit for predicting the outcome of sperm extraction for patients with non-obstructive azoospermia. The kit of the present invention includes a reagent for detecting the expression level of lncRNA in the sample, the lncRNA includes SPATA42, CCDC37-DT, GABRG3-AS1, LOC440934, LOC100505685, LOC101929088 (XR_927561.2), LOC101929088 (XR_001745218.1), LINC00343, LINC00343, LINC00343, LINC00343 at least one of them. The invention can realize the non-invasive and accurate prediction of the sperm retrieval outcome of NOA patients before operation, and has sufficient scientificity and practicability.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
Method for successful retrieval of sperm
ActiveUS10822657B2High degree of sensitivityStrong specificityMicrobiological testing/measurementBiological material analysisSemenBiology
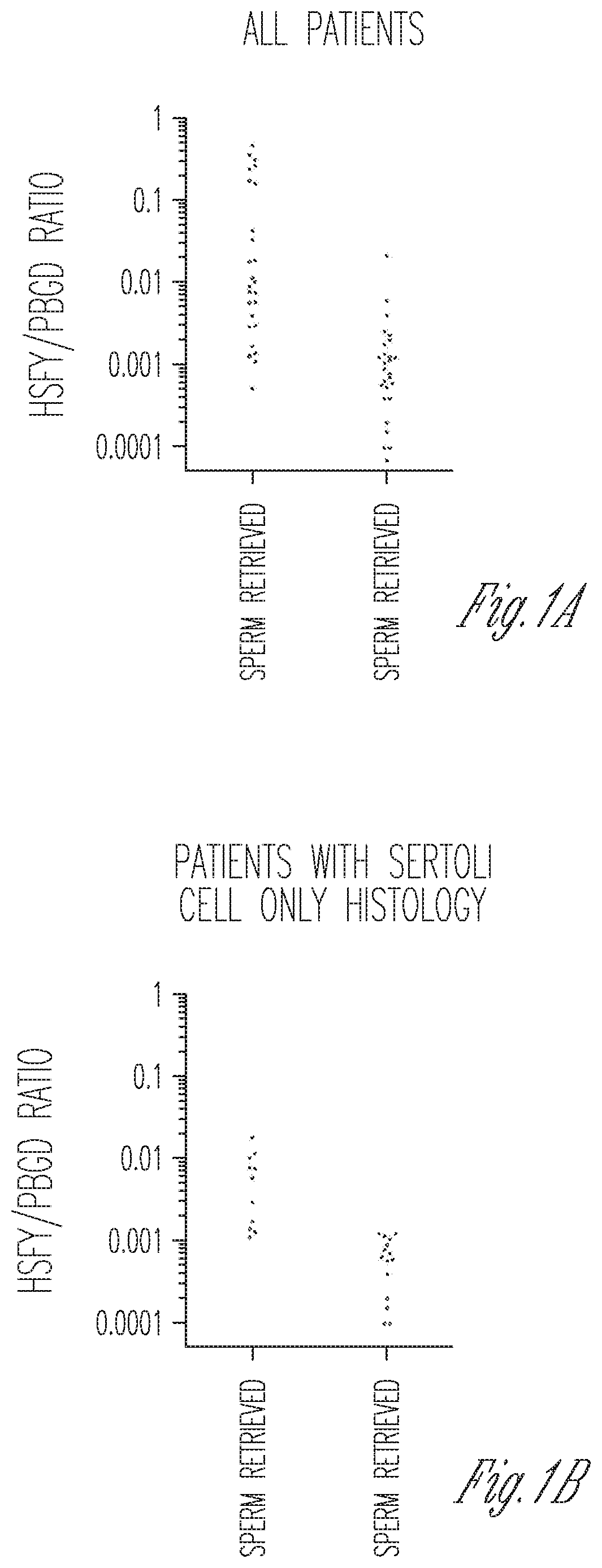
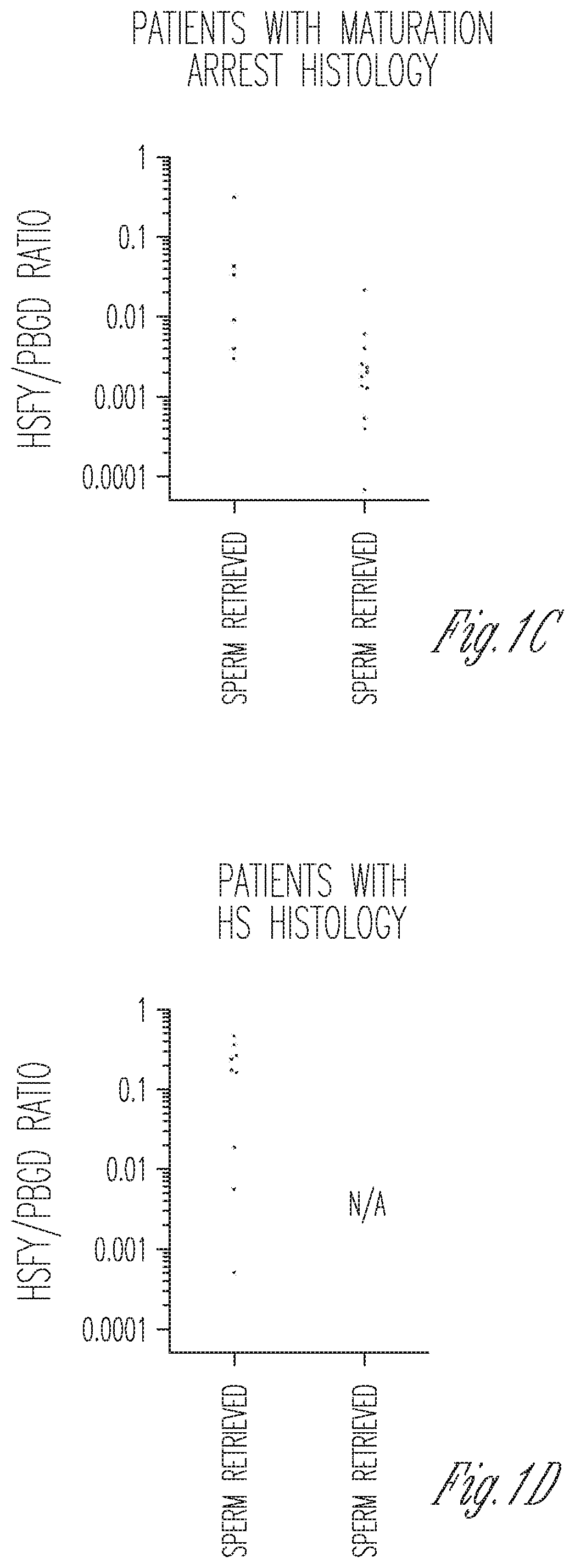
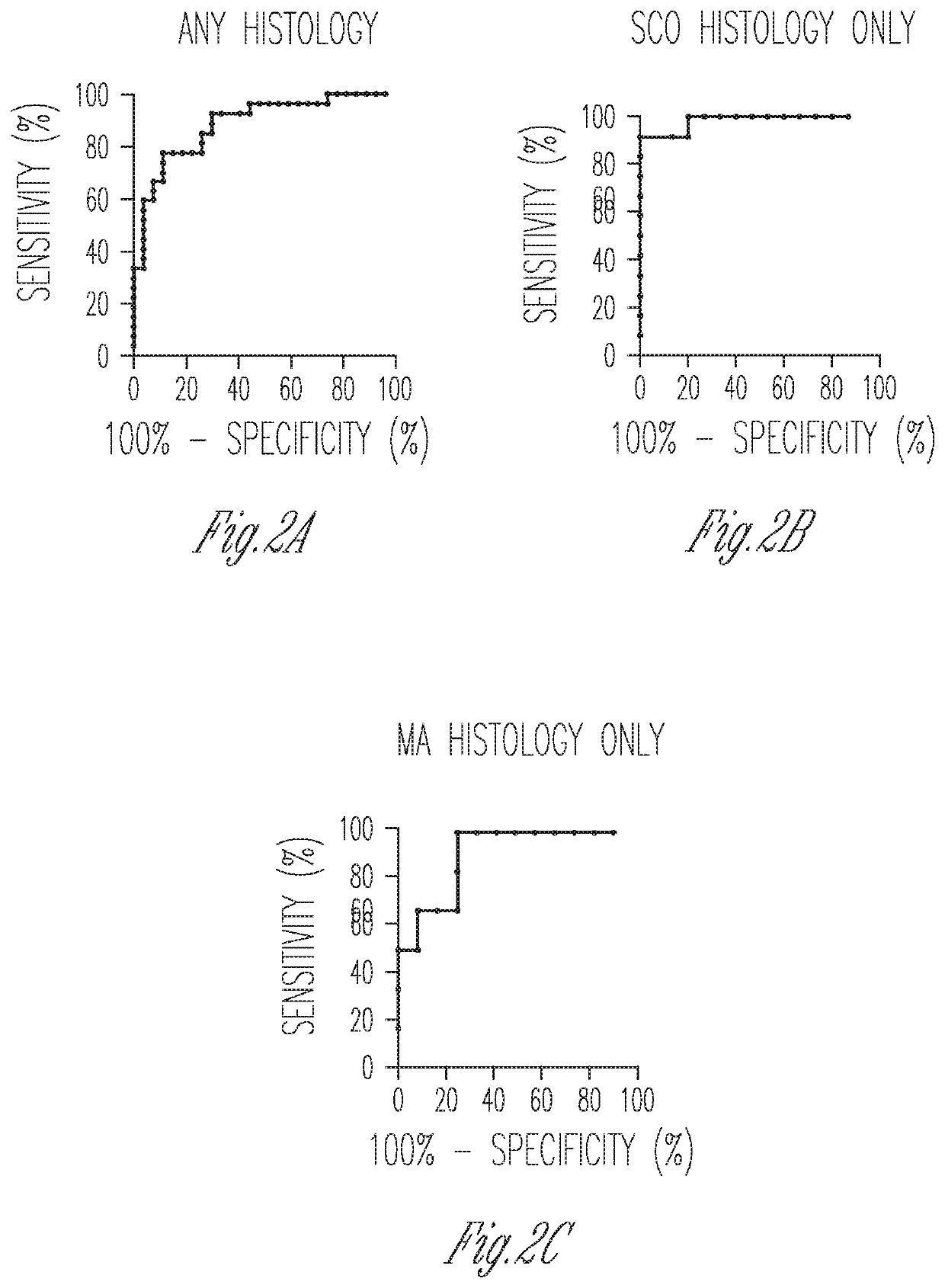
Measurement of expression levels of heat shock factor Y chromosome (HSFY) in testicular tissue samples or semen can be used to identify whether testicular sperm extraction can be used for patients with all histological variants of nonobstructive azoospermia.
Owner:CORNELL UNIVERSITY
Kit for evaluating testis microscopic sperm extraction effect of idiopathic non-obstructive azoospermia patient
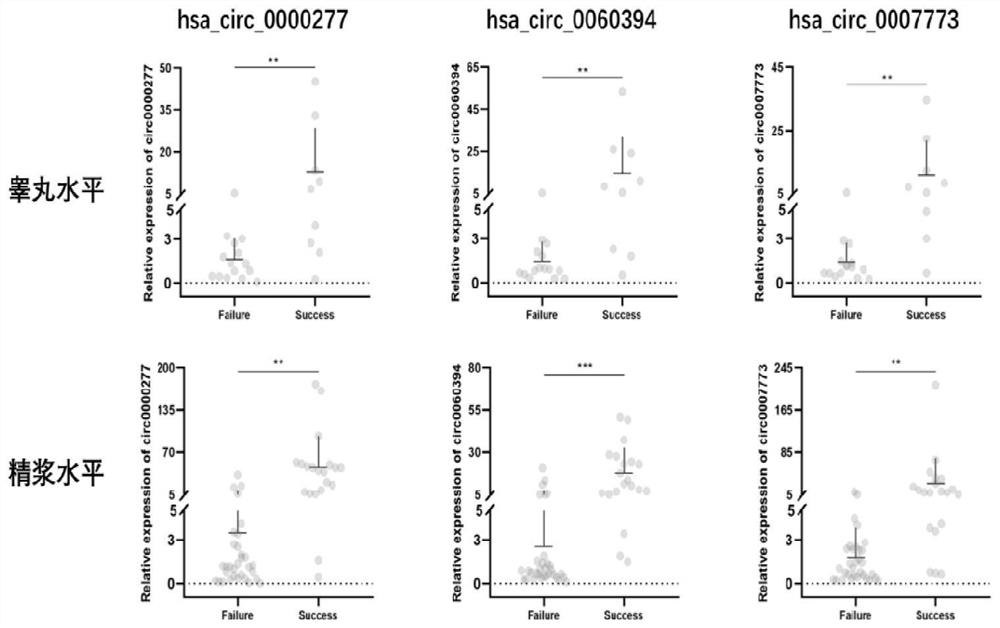
ActiveCN113584155AStable expressionEasy to get materialsMicrobiological testing/measurementAgainst vector-borne diseasesBiologySpermatid
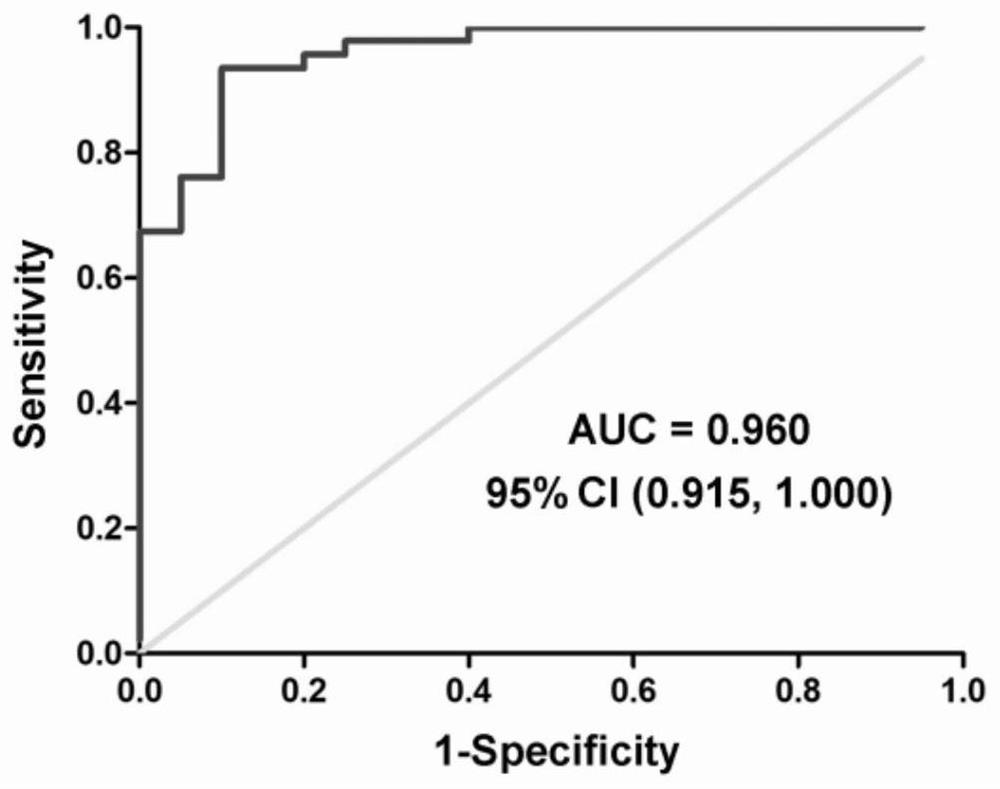
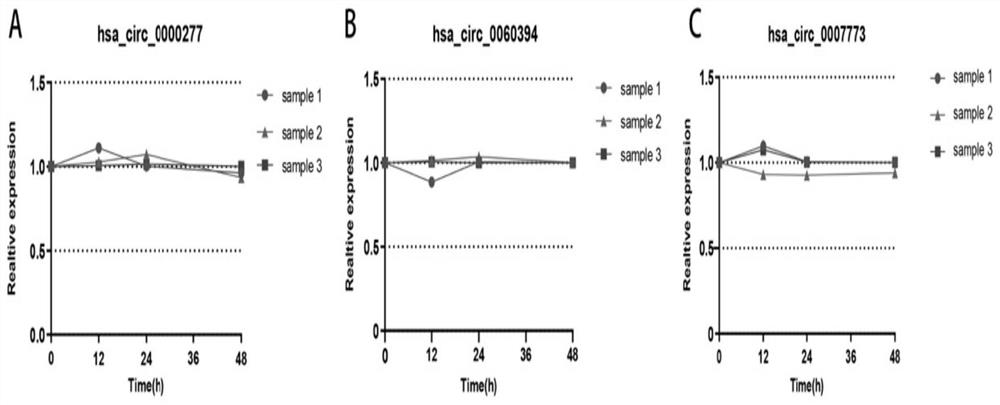
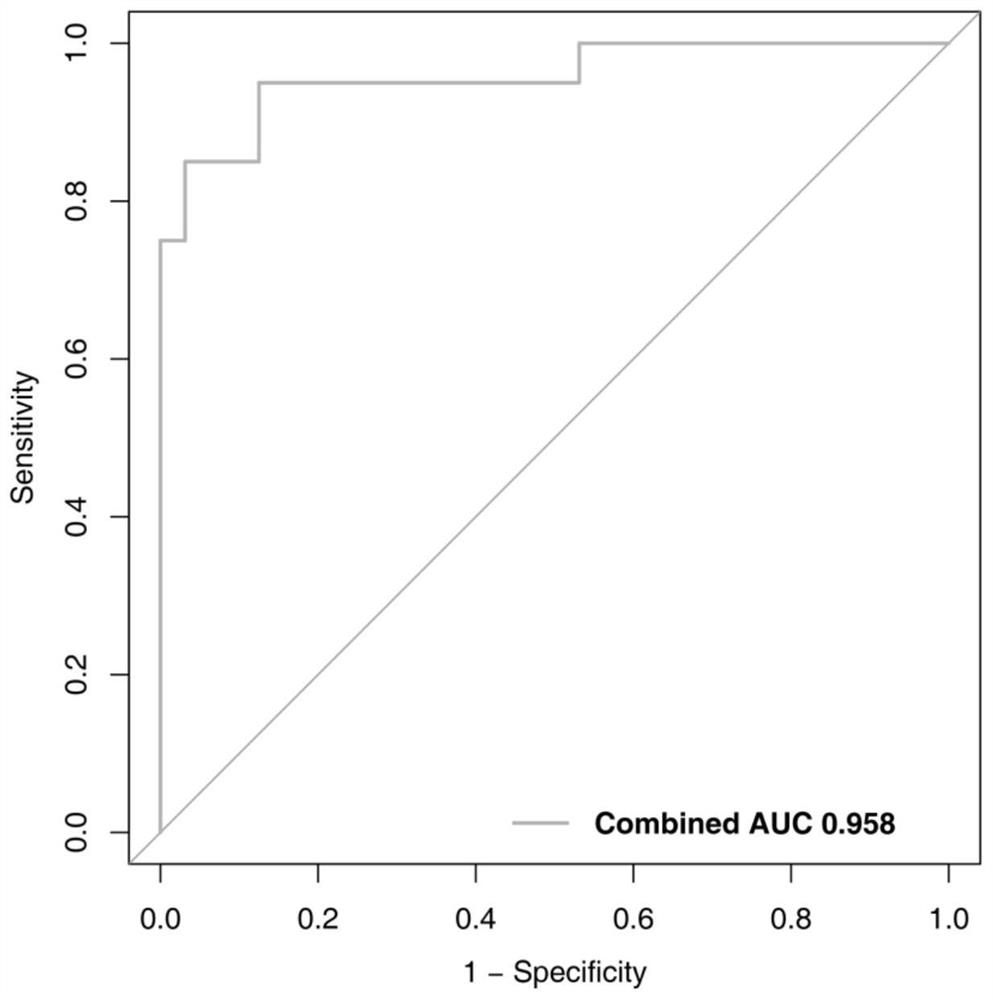
The invention belongs to the technical field of biology, and particularly relates to a kit for evaluating the testis microscopic sperm extraction effect of an idiopathic non-obstructive azoospermia patient, reagents contained in the kit are used for determining the content of three circRNAs, namely, hsa_circ_0000277, hsa_circ_0060394 and hsa_circ_0007773, in seminal plasma, the three circRNAs are combined through logistic regression to construct a model with a higher diagnostic value, and the kit is used for evaluating the testis microscopic sperm extraction effect of an idiopathic non-obstructive azoospermia patient. The kit has the beneficial effects that: the circRNA from the testis in the seminal plasma is stable in expression and convenient to obtain, and can be used for dynamically reflecting the spermatogenic state in the testis. Clinical specimens verify that circRNA from testis in seminal plasma has high diagnostic value as an evaluation marker of microscopic sperm extraction effect of idiopathic NOA patients.
Owner:JIANGSU PROVINCE HOSPITAL THE FIRST AFFILIATED HOSPITAL WITH NANJING MEDICAL UNIV +1
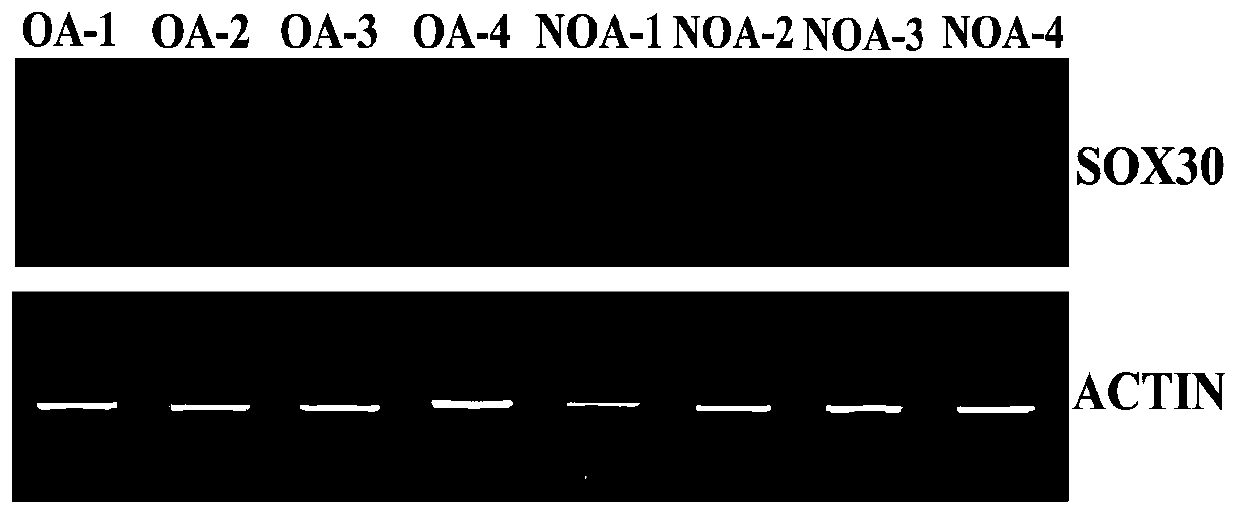
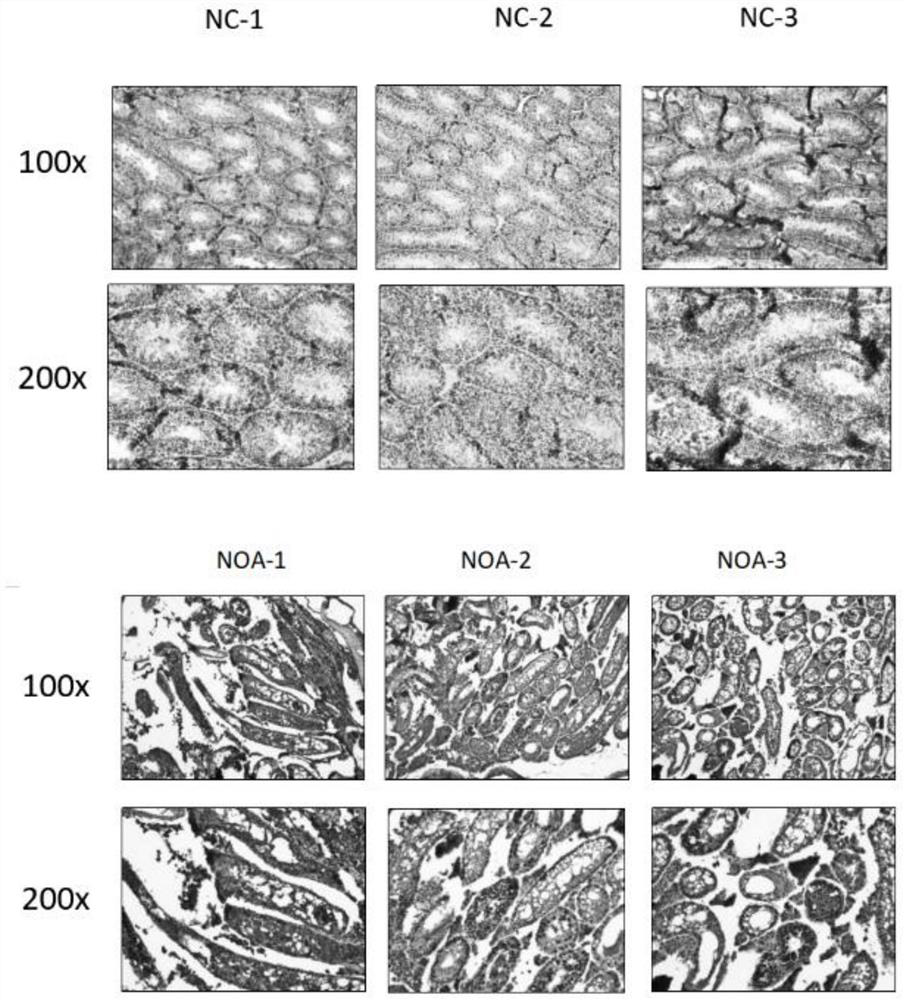
Diagnosis box for non-obstructive azoospermia (NOA)
ActiveCN110577991AUnderstand the causeImprove accuracyMicrobiological testing/measurementDisease diagnosisTesticleBiology
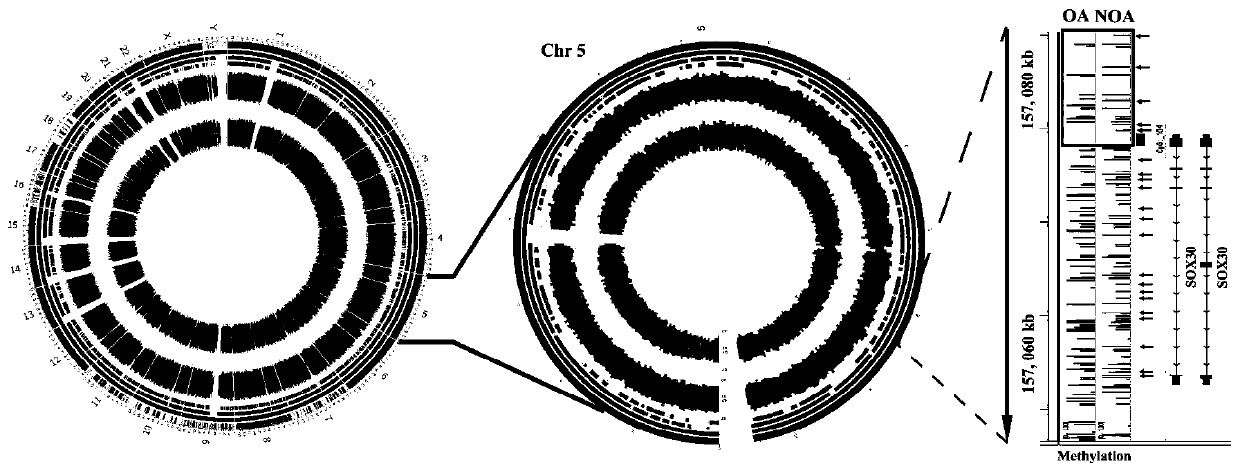
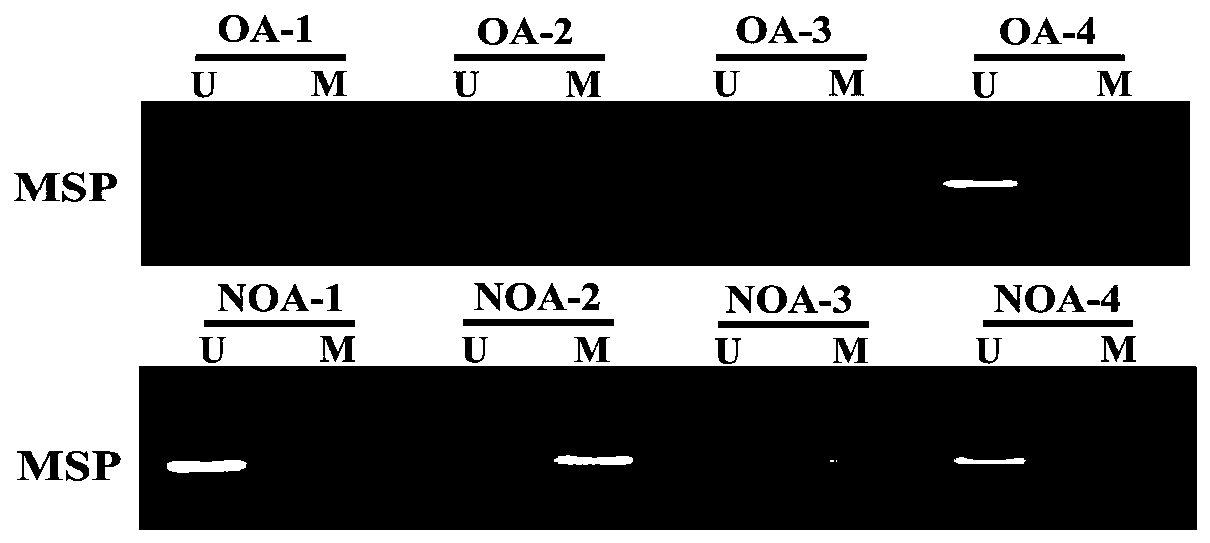
The invention discloses a diagnosis box for non-obstructive azoospermia (NOA). According to the diagnosis box, the methylation states of SOX30 genes and the expression levels of mRNA and coding proteins in naturally compared obstructive azoospermia (OA) and NOA punctured testis tissue are detected. The detection result shows that the SOX30 genes are subjected to specific hypermethylation and lossof expression in the NOA punctured testis tissue. Based on the detection result, the invention provides the diagnostic box for NOA, clinical NOA patients can be helped to formulate effective treatmentschemes to improve precision and accelerate the treatment progress, and meanwhile the diagnosis box has important significance for better understanding of NOA nosogenesis, formulating risk assessmentand early diagnosis.
Owner:ARMY MEDICAL UNIV
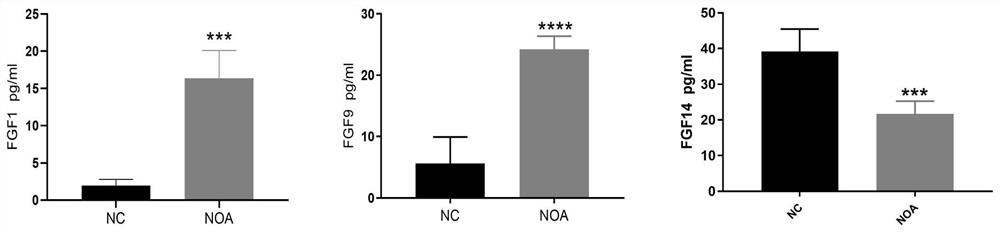
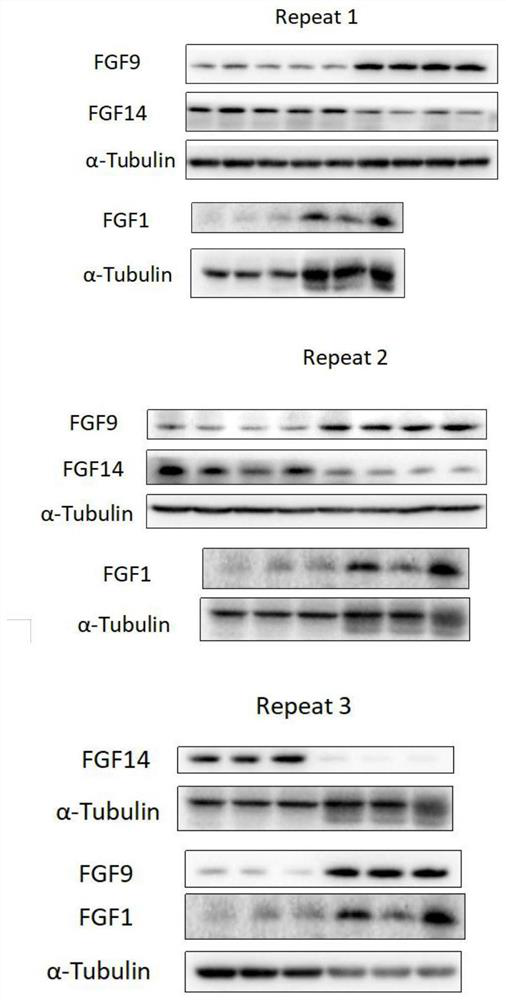
Biomarker combination for diagnosing non-obstructive azoospermia and application thereof
PendingCN113820498AStrong specificityImprove accuracyDisease diagnosisBiological testingAssayBiologic marker
The invention provides a biomarker combination for diagnosing non-obstructive azoospermia. The biomarker combination comprises FGF1 (fibroblast growth factor 1), FGF9 (fibroblast growth factor 9) and FGF14 (fibroblast growth factor 14), the biomarker combination can be used for preparing an ELISA (enzyme-linked immunosorbent assay) detection kit, the non-obstructive azoospermia is diagnosed by detecting expression conditions of FGF1, FGF9 and FGF14 in a blood sample of a patient, the pain of the patient can be greatly relieved, and the application prospect is wide.
Owner:温州医科大学慈溪生物医药研究院
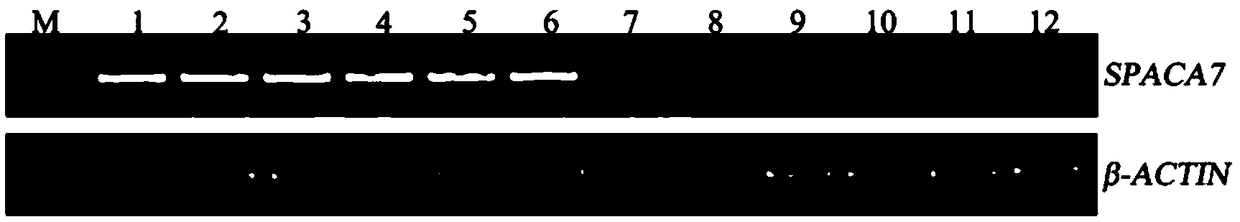
Application of SPACA7 gene
InactiveCN108866178AEasy to operateShort timeMicrobiological testing/measurementAgricultural scienceAzoospermia
The invention discloses application of an SPACA7 gene. The SPACA7 gene is used for preparing a product for detecting non-obstructive azoospermia. The product comprises a pair of primers for specifically amplifying the SPACA7 gene; the sequence of an upstream primer is shown as SEQ ID NO: 1 and the sequence of a downstream primer is shown as SEQ ID NO: 2. The SPACA7 gene provides a new detection index for detecting the non-obstructive azoospermia. The distinctive detection between normal bearers and azoospermia patients can be achieved by using only a pair of primers through the RT-PCR detection; the whole operation process is simpler, consumes shorter time and is beneficial to clinical testing.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
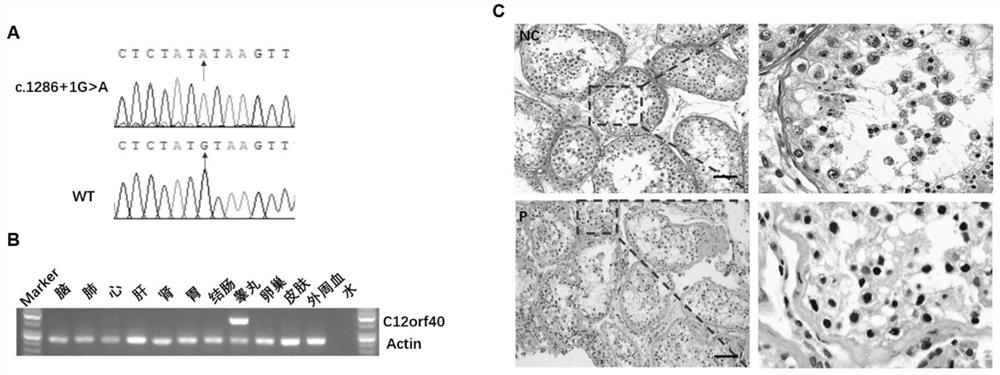
Application of C12orf40 gene
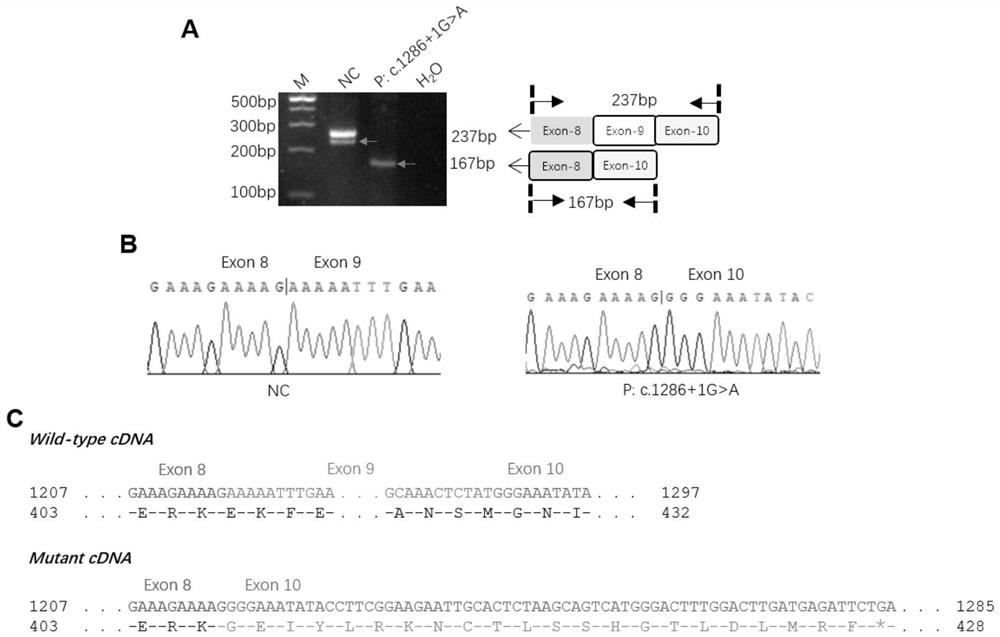
PendingCN114875127AMicrobiological testing/measurementDisease diagnosisDiseaseObstructive azoospermia
The invention relates to an application of a C12orf40 gene. In clinical scientific research and practice, the inventor screens and identifies a disease-causing gene C12orf40 cleavage site variation (NM001031748.4, c.1286 + 1Ggt; a), no haploid sperms are found in testis biopsy tissues of a patient, and non-obstructive azoospermia is shown. The gene is specifically expressed in testis tissues, is not expressed in other tissues, can be used as a biomarker of the non-obstructive azoospermia, and is beneficial to further research on a mechanism of the non-obstructive azoospermia and exploration on treatment of the non-obstructive azoospermia.
Owner:REPRODUCTIVE & GENETIC HOSPITAL OF CITIC XIANGYA CO LTD +1
A seminal exosomal tsRNA marker associated with the diagnosis of non-obstructive azoospermia and its application
ActiveCN112251508BEasy diagnosisImprove discriminationMicrobiological testing/measurementDNA/RNA fragmentationAzoospermiaBlood plasma
The invention discloses a seminal plasma exosome tsRNA marker related to the diagnosis of non-obstructive azoospermia and its application. The markers are tRF-Pro-AGG-003 and tRF-Val-AAC-010, the sequence of tRF-Pro-AGG-003 is SEQ ID NO.1, and the sequence of tRF-Val-AAC-010 is SEQ ID NO. 2. The marker has specificity and sensitivity to non-obstructive azoospermia, and can be used to prepare a diagnostic kit for non-obstructive azoospermia. The two seminal plasma exosome markers tRF-Pro-AGG-003 and tRF-Val-AAC-010 of the present invention are differentially expressed in the seminal plasma of patients with non-obstructive azoospermia and obstructive azoospermia, which can be compared with It can diagnose non-obstructive azoospermia well, and can predict the result of sperm retrieval in azoospermia, and its effect is better than commonly used indicators such as plasma follicle stimulating hormone level and testicular volume.
Owner:江苏阔然生物医药科技有限公司
Biomarker for detecting non-obstructive azoospermia and application thereof
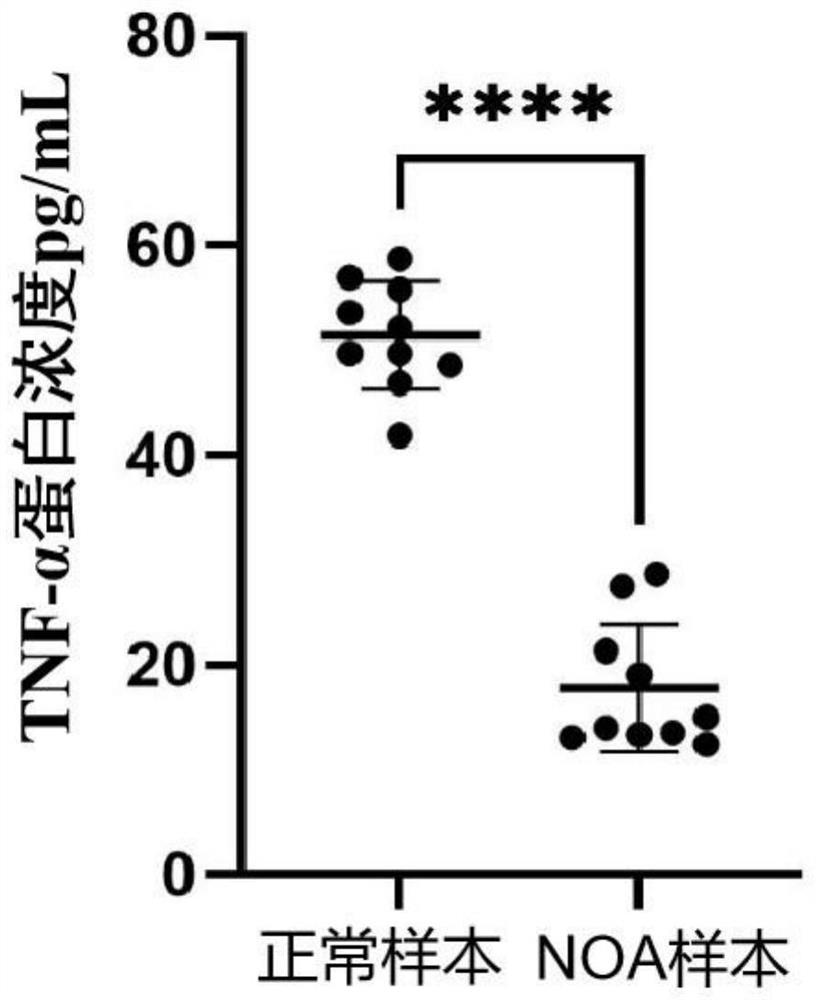
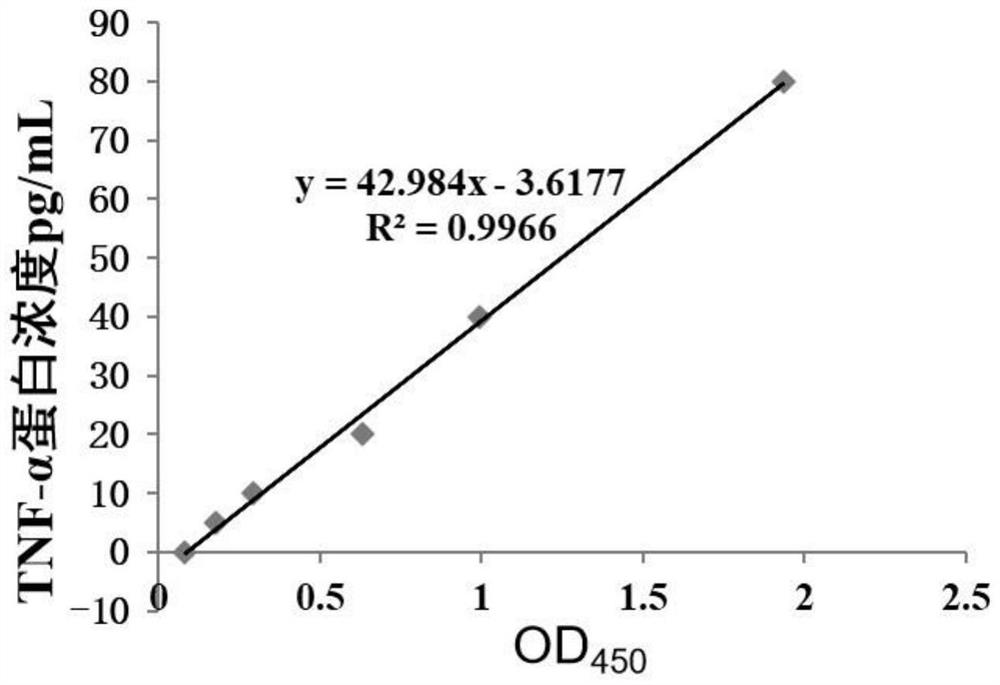
The invention discloses a biomarker for detecting non-obstructive azoospermia and application of the biomarker. The biomarker comprises a TNF-alpha (tumor necrosis factor-alpha) protein. The invention finds that the concentration of the Wnt receptor inhibitory protein TNF-alpha in the semen of the NOA patient is obviously lower than that of a normal male for the first time, and proposes that the TNF-alpha protein can be used as a potential early warning molecule for evaluating the spermatogenic dysfunction of the NOA patient, is beneficial to expansion and diagnosis of NOA indexes so as to expand the diagnosis range, and has important significance in the field of NOA diagnosis.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV
A plasma exosomal tsRNA marker associated with the diagnosis of non-obstructive azoospermia and its application
ActiveCN112176052BPrediction of sperm retrieval resultsEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationTesticular volumeBlood plasma
The invention discloses a plasma exosome tsRNA marker related to the diagnosis of non-obstructive azoospermia and its application. The marker is tRF-Gly-GCC-002, and the sequence of tRF-Gly-GCC-002 is SEQ ID NO.1. The marker has specificity and sensitivity to non-obstructive azoospermia, and can be used to prepare a diagnostic kit for non-obstructive azoospermia. The plasma exosome marker tRF-Gly-GCC-002 of the present invention is differentially expressed in the plasma of patients with non-obstructive azoospermia and obstructive azoospermia, which can better diagnose non-obstructive azoospermia, and can The effect of predicting the result of sperm retrieval in azoospermia is better than the commonly used indicators such as plasma follicle stimulating hormone level and testicular volume.
Owner:XUZHOU MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com