Method for preparing novel oncolytic virus by exosome-like technology
A technology of oncolytic virus and exosomes, which is applied in the field of oncolytic virus preparation to achieve high targeting, improve efficacy, and reduce side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
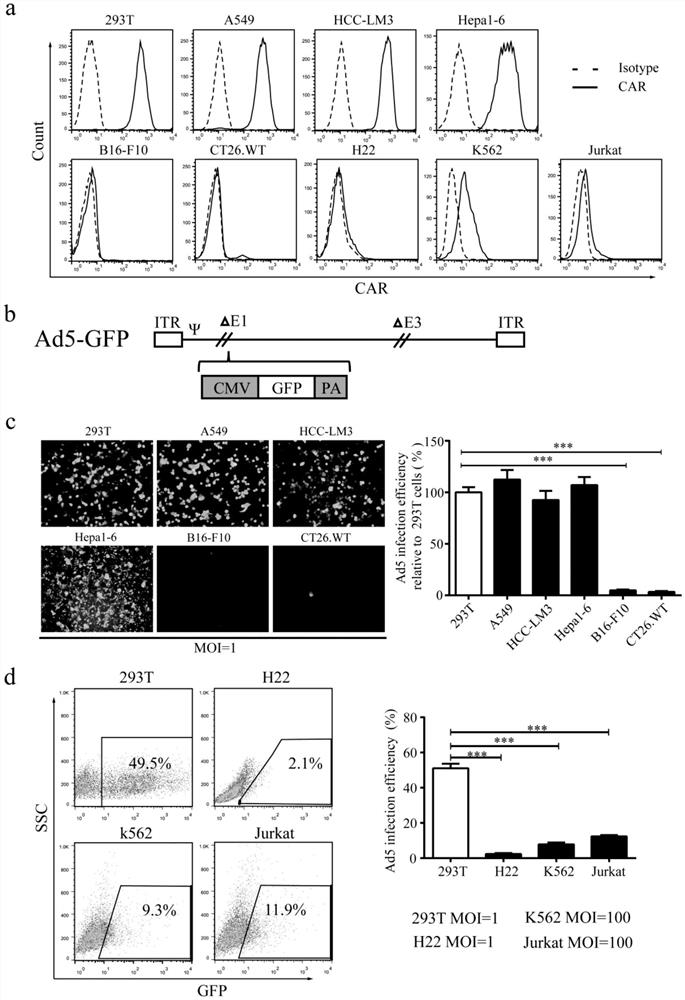
[0055] Example 1 Infection efficiency of adenovirus Ad5 to cells with low CAR expression is low
[0056] CAR (the coxsackie and adenovirus receptor) is the main receptor of adenovirus Ad5 infected cells. Early studies have demonstrated that adenovirus Ad5 has low infection efficiency for cells with low CAR expression. 13,19 We found that the fluorescence intensity of 293T, A549, HCC-LM3 and Hepa1-6 cells stained with anti-CAR-PE was significantly increased compared with the isotype treatment group by flow cytometric analysis, indicating that 293T, A549, HCC-LM3 and Hepa1-6 High expression of CAR on the cell surface ( figure 1 a); while the surface of B16-F10, CT26.WT and H22 cells were stained with CAR antibody, there was no significant change in fluorescence intensity compared with the isotype treatment group; indicating that these cell lines do not express CAR; while K562 and Jurkat cells were stained with CAR antibody After that, compared with the isotype treatment group...
Embodiment 2
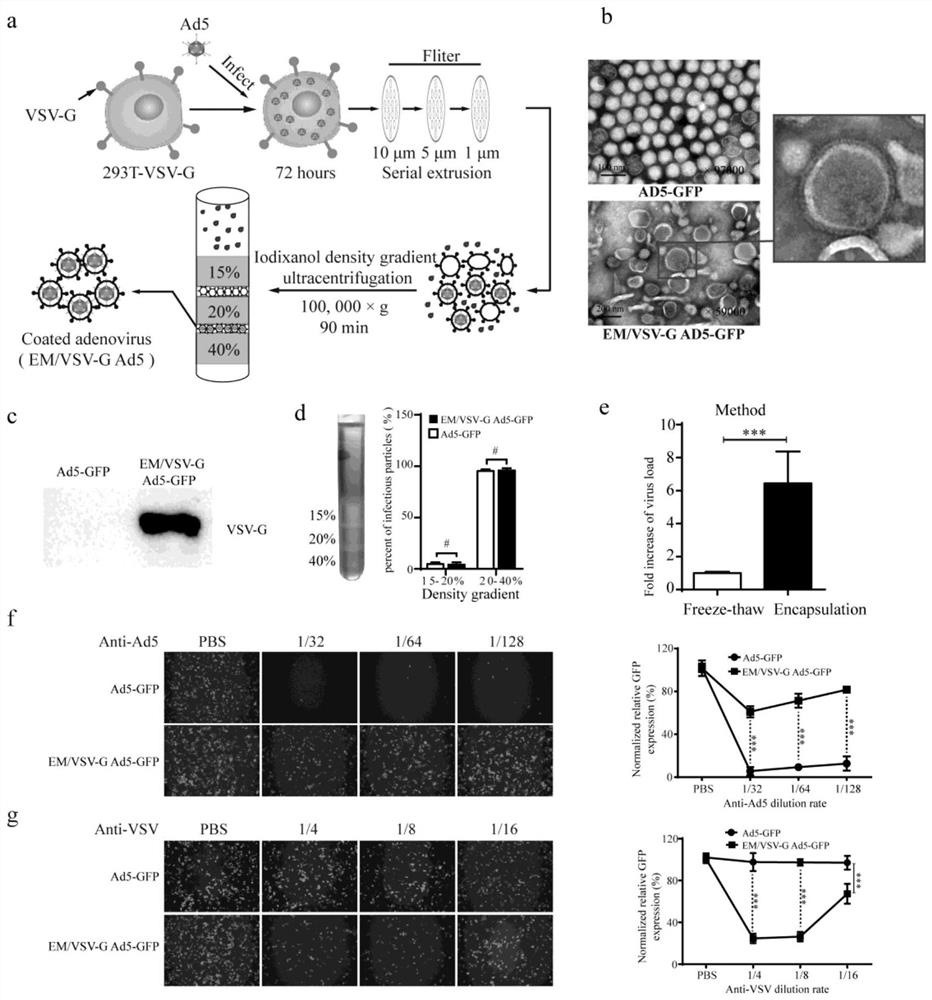
[0057] Example 2 Preparation of Exosome-mimetic Ad5 (EM / VSV-G Ad5) with VSV-G
[0058] Vesicular stomatitis Indiana virus G protein (VSV-G) is capable of mediating viral entry into all cell types tested so far and is widely used in gene transduction and gene therapy 26 . This study hopes to use Exosome-mimetic technology to take advantage of the broad tropism of VSV-G on cells to realize the retargeting of Ad5 and increase the infection efficiency of Ad5 on CAR low-expression cell lines.
[0059] First, we constructed 293T cells expressing VSV-G (293T-VSV-G), infected 293T-VSV-G cells with Ad5-GFP at an MOI of 5, cultured at 37°C for 72 hours, and collected the cells. The collected cells were divided into two parts, Part of the virus was purified by iodixanol density gradient centrifugation after freezing and thawing 3 times using the traditional protocol; Squeeze sequentially through polycarbonate membranes with pore sizes of 10 μm, 5 μm, and 1 μm, using 15%, 20%, and 40% i...
Embodiment 3
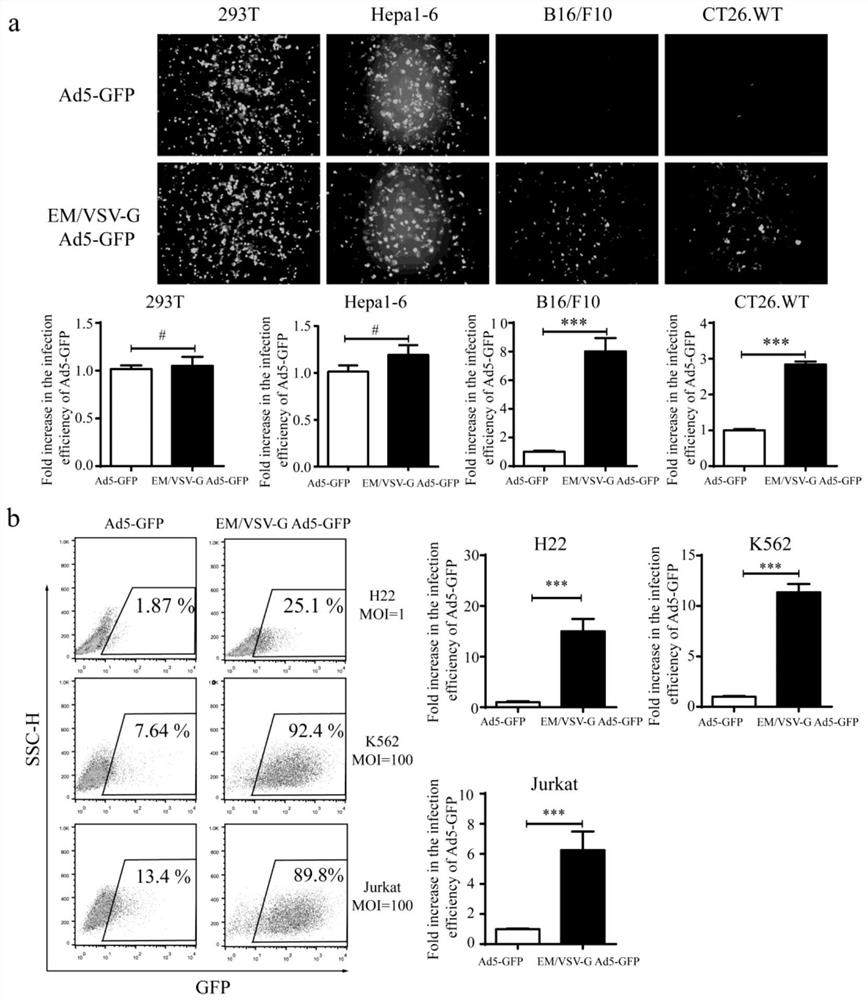
[0061] Example 3 EM / VSV-G Ad5-GFP can resist the effect of anti-Ad5 neutralizing antibody and the carried VSV-G mediates its entry into cells
[0062] To further prove that EM / VSV-G Ad5-GFP is encapsulated in vesicles, and that such encapsulation brings new properties, we used anti-Ad5 neutralizing antibody and VSV-G neutralizing serum for EM / VSV- G Ad5-GFP was processed for subsequent analysis. After treating EM / VSV-G Ad5-GFP and Ad5-GFP viruses with anti-Ad5 neutralizing antibodies at dilutions of 1 / 32, 1 / 64, and 1 / 128, the infection efficiency of Ad5-GFP virus was only that of the PBS-treated control group 5.66±2.1%, 9.3±2.02% and 12.6±3.84%, while the infection efficiency of EM / VSV-G Ad5-GFP was 61.0±3.10%, 71.3±3.7% and 81.6±1.2% ( figure 2 f) It can be seen that after treatment with anti-Ad5 neutralizing antibody, the Ad5-GFP virus almost lost its ability to infect, while the EM / VSV-G Ad5-GFP was infected by the exosome-like outer membrane and the presence of VSV-G. Ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com