Novel hyaluronase variants with improved stability and pharmaceutical compositions containing same
A composition and variant technology, applied in the field of novel human PH20 variants or fragments thereof, can solve the problems of reducing the stability and deficiency of protein drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
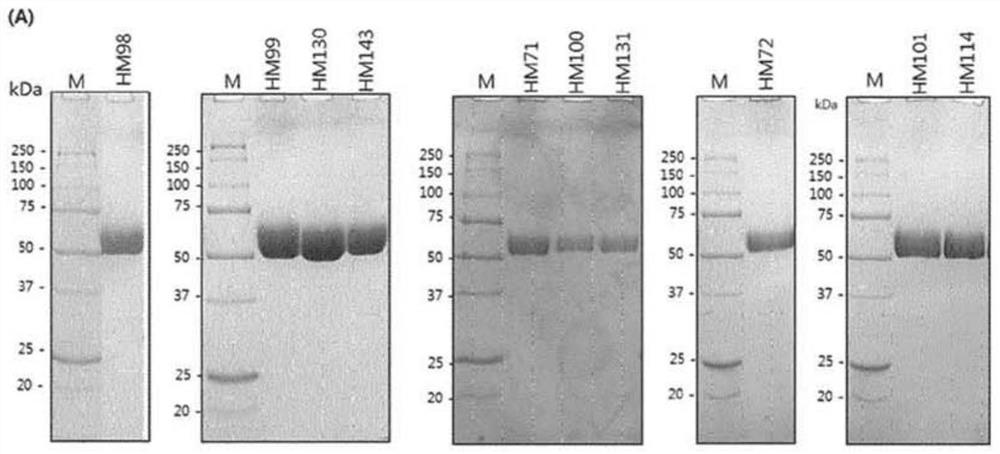
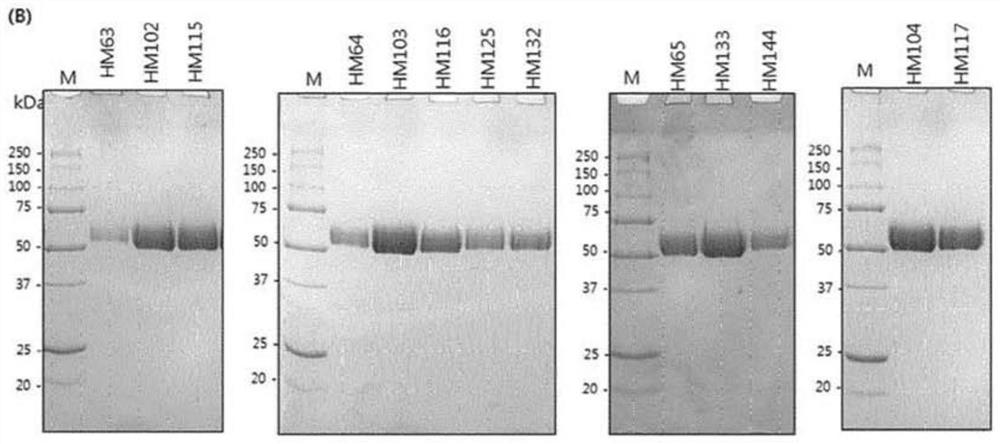
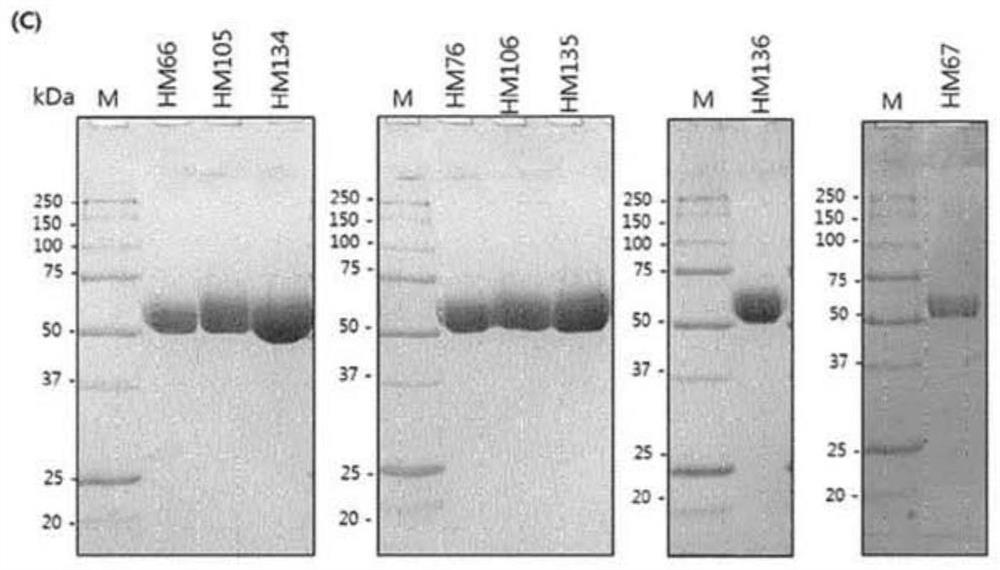
[0127] Example 1: Construction of PH20 variants
[0128] To construct PH20 variants, the cDNA of wild-type PH20 (clone ID: hMU002604) was purchased from the Korean Human Gene Bank. Wild-type PH20 encodes amino acids from L36 to S490. The PH20 gene was amplified by polymerase chain reaction (hereinafter referred to as PCR) and inserted into the restriction enzyme sites XhoI and NotI of the pcDNA3.4-TOPO vector. For expression in ExpiCHO cells, the signal peptide of human growth hormone, human serum hormone or human Hyal1 was used as a signal peptide instead of the original signal peptide of PH20. For protein purification using HisTrap columns, the DNA sequence of the His-tag is located at the 3' end of the cDNA of PH20. Amino acid substitutions of the PH20 variants were made using PCR and confirmed by DNA sequencing.
[0129] The list of primers used to clone PH20 variants is summarized in Table 4 below, and the specific primer sequences are summarized in Table 5 below.
[...
Embodiment 2
[0236] Example 2: Properties of PH20 variants according to the invention
[0237] The structure and function of the protein were further investigated by studying variants comprising N-terminal and C-terminal cleavages based on the amino acid sequence of SEQ ID NO:3. The aggregation temperature shown in Table 7 is used as the analysis result of the performance and activity of the prepared variants.
[0238] The extent of expression and specific activity was analyzed by turbidity assay as described in Example 1. The results of the test are shown below. At this time, based on the limit of quantification (LOQ) set for each activity after purification in the culture medium, an activity exceeding 300 unit / mL in the culture medium is marked as ">LOQ", while an activity exceeding 15 unit after purification / μg is marked as ">LOQ". In the opposite case, the sign of the inequality changes. The performance level and quantification limit of the activity analysis and the test results b...
Embodiment 3
[0250] Example 3: Activity analysis of variants substituted with the sequences Hyal2, Hyal3 and Hyal4
[0251] The amino acid sequences of Hyal2 (TTSTETCQYLKDYLTRL), Hyal3 (SSSEEECWHLHDYLVDT) and Hyal4 (TASKANCTKVKQFVSSD) are the corresponding parts of hyaluronidases in the human body except Hyal1, replacing the amino acid sequence of M345 to I361 in the wild-type PH20 of SEQ ID NO:1 position to study how the stability of the protein changes.
[0252] The variants constructed by substituting the corresponding sequences Hyal2, Hyal3 and Hyal4 for the positions from M345 to I361 of mature wild-type PH20 (L36-S490) are referred to as "Hyal2-variant", "Hyal3-variant" and "Hyal3-variant", respectively. Hyal4-variant".
[0253] Hyal2-variant, Hyal3-variant and Hyal4-variant were constructed, then the thermostability of these variants was analyzed (see figure 2). As a result, the aggregation temperature of the Hyal2-variant measured by DLS was 48°C, which was 1.5°C higher than the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com