Ultrabright nir-ii aiegen for bioimaging
A technology of aggregation-induced luminescence and compounds, applied in the field of fluorophores
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] synthesis
[0135] Compound 2TT-oC6B :
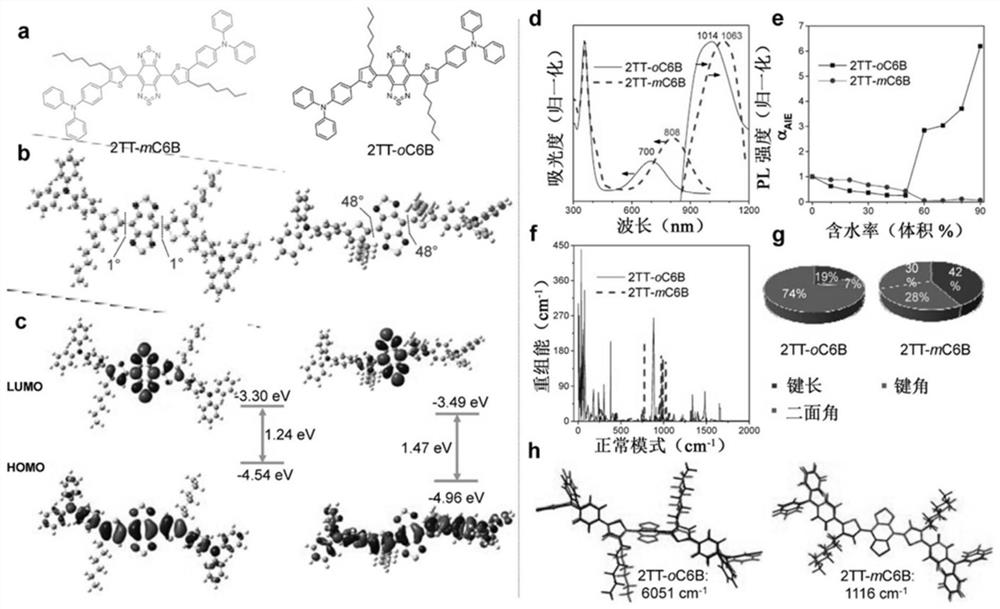
[0136] To synthesize compound 2TT-oC6B, organotin (0.7 g, 1 mmol), dibromo-BBT (87 mg, 0.25 mmol), Pd 2 (dba) 3 (22mg, 0.025mmol), P(o-tol) 3 (66 mg, 0.21 mmol) and degassed dry toluene (1.5 mL) and seal with a Teflon cap. in N 2 Under atmosphere, the reaction mixture was heated to 130 °C and stirred for 48 h. After cooling, the crude product was quenched with KF solution and extracted with DCM. use Na 2 SO 4 The combined organic phases are dried. After removing the solvent, the product was purified with a silica gel column to obtain a dark green solid (yield: 35%). 1 H NMR (400MHz, CDCl 3 ),δ(ppm)=7.59-7.56(4H,m),7.37(2H,s),7.31-7.26(8H,m), 7.16-7.12(8H,m),7.11-7.03(8H,m), 2.61-2.57(4H,t,J=8Hz),1.63,(4H,m),1.15-1.10(12H,m),0.73(6H,m). 13 C NMR (100MHz, CDCl 3 ), δ (ppm): 152.6, 146.9, 146.8, 146.3, 145.0, 128.7, 127.5, 126.1, 124.1, 124.0, 122.7, 122.5, 115.4, 99.3, 30.8, 29.8, 29.6, 28.4, 21.8, 13.3. / z: [M]+C...
Embodiment 2
[0138] Determination of the fluorescence quantum yield (QY) of the dye.
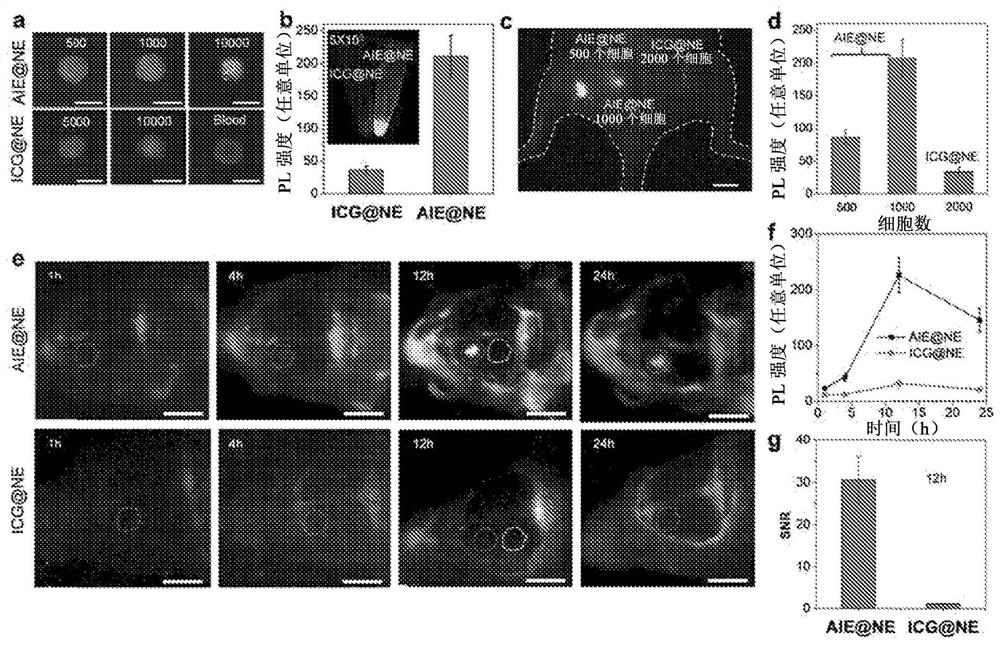
[0139] The QY of the dye was determined (QY = 0.5%) using the NIR-II fluorescent IR-26 dye as a reference. For reference calibration, IR-26 dissolved in 1,2-dichloroethane (DCE) was diluted to form a DCE solution to prepare absorbance values at 808 nm of ~0.1, ~0.08, ~0.06, ~0.04, and ~0.02 for five samples, as these highly diluted samples minimize secondary optical processes such as reabsorption and reluminescence effects. Then, a total of five concentrations of linearly spaced DCE solutions of IR-26 were transferred at once into fluorescent tubes with a path of 10 mm. The excitation source is a diode laser of 808nm. Luminescence was collected in transmission geometry with a 900nm longpass filter to block excitation light, and emission spectra were taken in the 900nm to 1500nm region. DCE and H for ACQ and AIE dyes 2 O solution for the same operation. Then, the entire emission spectrum of both ...
Embodiment 3
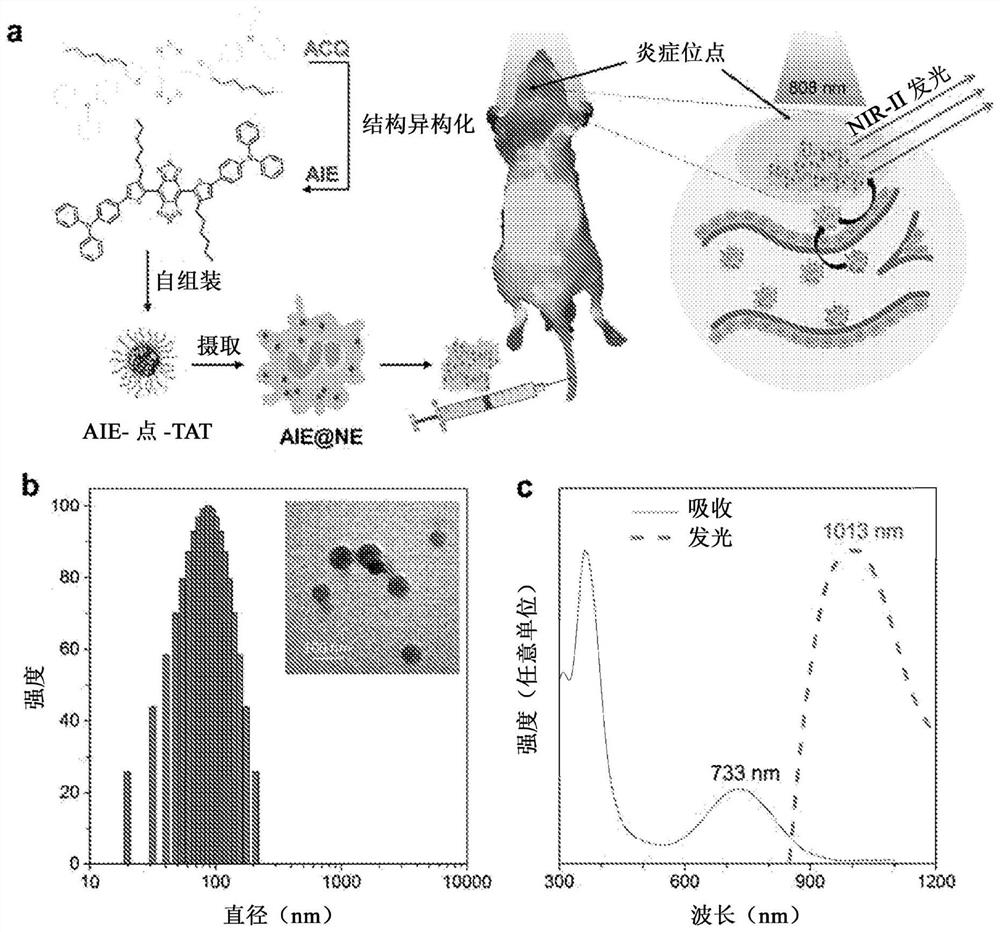
[0143] Production of AIE points
[0144] A mixture of 2TT-oC6B (1 mg), DSPE-PEG2000-maleimide (1.5 mg) and THF (1 mL) was sonicated (output 12W, XL2000, Misonix Incorporated, NY) to obtain a clear solution. The mixture was quickly injected into 9 mL of water, and vigorously sonicated in water for 2 min. The mixture was stirred for 12 h in smoked food to remove THF. The AIE point suspension was subjected to ultrafiltration at 3000 g for 30 min (molecular weight cut-off 100 kDa). The amount of 2TT-oC6B aggregates successfully encapsulated into the DSPE-PEG2000-maleimide matrix was estimated by absorbance spectra using the calibration curve of 2TT-oC6B in THF solution as reference.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com