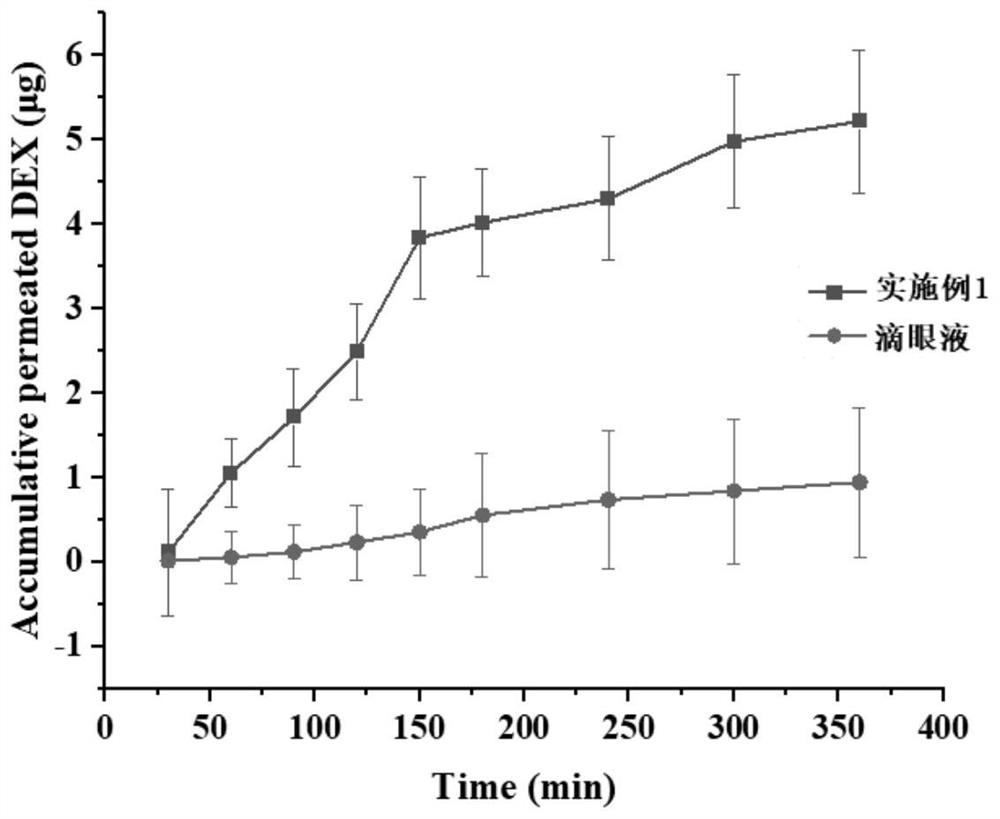
Dexamethasone in-situ liquid crystal gel preparation for treating diabetic retinopathy and preparation method and application thereof
A technology for diabetic retina and dexamethasone, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, metabolic diseases, etc., can solve problems such as short half-life, achieve high long-term stability, improve drug biological Availability, the effect of reducing the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A method for preparing dexamethasone in situ liquid crystal gel preparation, comprising the following steps:
[0031] (1) Accurately weigh 65 mg of phytantriol and heat in a water bath at 60° C. as the oil phase.
[0032] (2) Precisely weigh 1 mg of dexamethasone, 30 mg of PEG400, and 5 μL of water for injection and heat in a water bath until the drug dexamethasone is dissolved as the water phase.
[0033] (3) Add the water phase to the oil phase, and quickly vortex it until it is colorless and transparent after adding, to obtain the dexamethasone in-situ liquid crystal gel preparation. Liquid crystal gels are liquid and semi-solid gels before and after in-situ (phase transition).
Embodiment 2
[0035] Phase inversion properties of dexamethasone in situ liquid crystal gel
[0036] Minimum artificial tear volume for phase inversion (V m ) and time (T g )
[0037] Take the dexamethasone in situ liquid crystal gel prepared in Example 1 for the experiment of phase inversion properties. Determination of the V of the formulation using the tube inversion method m and T g . Accurately weigh 200 mg of dexamethasone in-situ liquid crystal gel in a centrifuge tube. In order to simulate the human body environment, place the sample in a 37°C water bath, add 20 μL of artificial tears in sequence, vortex quickly, and tilt the sample at 90° to observe whether the sample is gelling. Complete gelation of the sample was demonstrated when no flow was observed. When the preparation is completely gelled, record the total amount of artificial tears added at the end of the experiment as V m , record the gel formation time as T g .
[0038] When the precursors are exposed to artific...
Embodiment 3
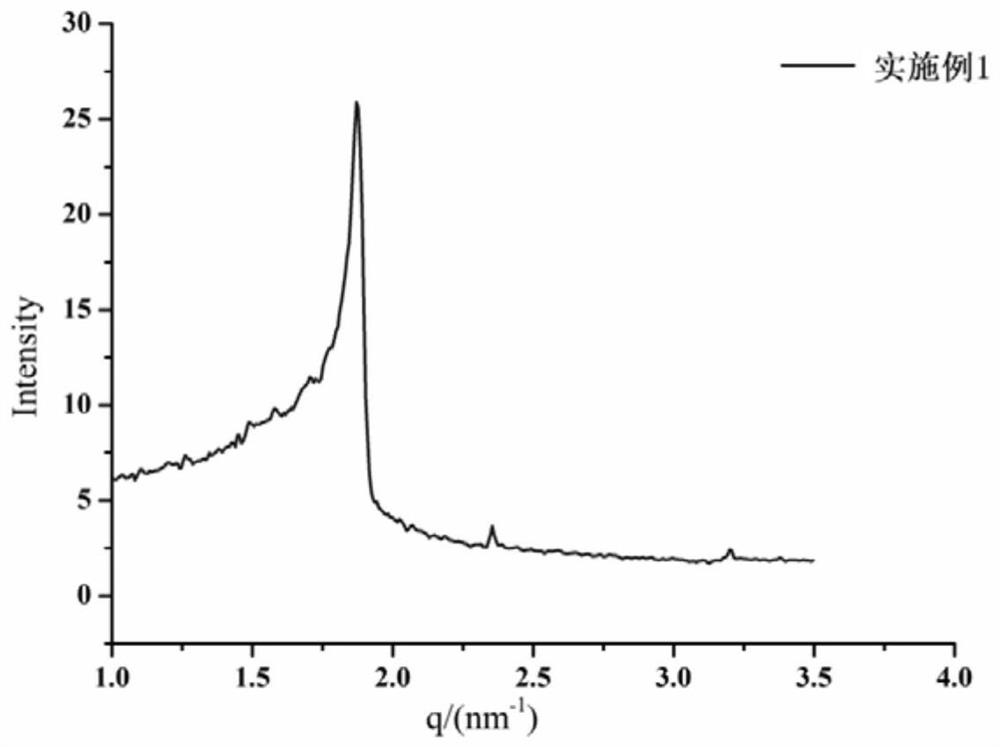
[0040] Polarizing microscope observation of dexamethasone in situ liquid crystal gel
[0041] The dexamethasone in situ liquid crystal gel prepared in Example 1 was taken for polarizing microscope observation. In order to investigate the internal morphology of the gel, a small amount of sample was placed on a glass slide and covered with a cover glass, and then observed under a polarizing microscope at room temperature. The results are as follows: figure 1 As shown, both before and after in situ (phase transition) of dexamethasone liquid crystal gel are in dark field.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com