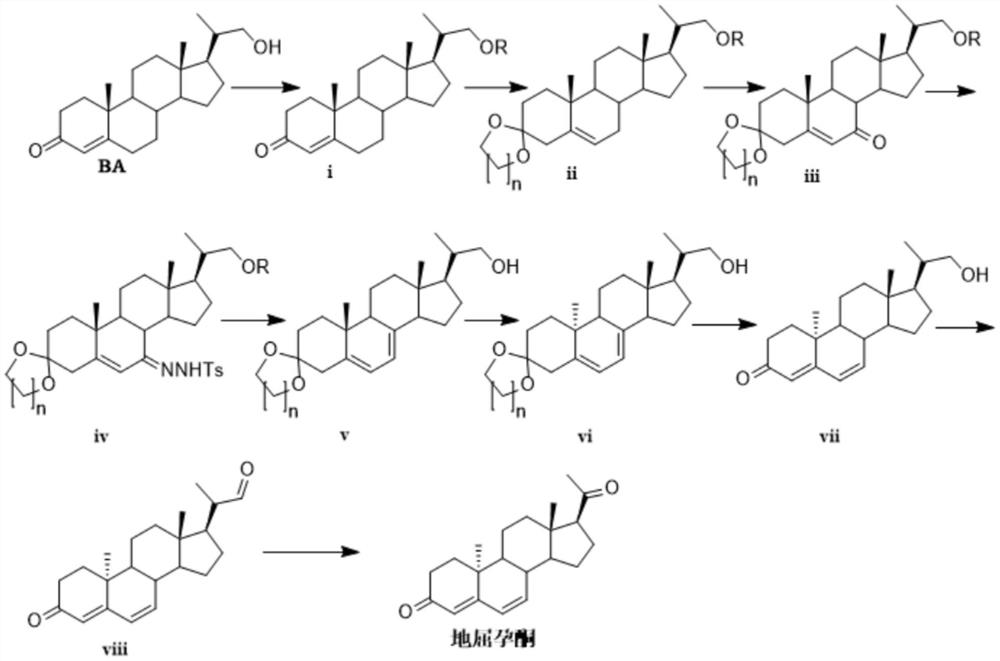
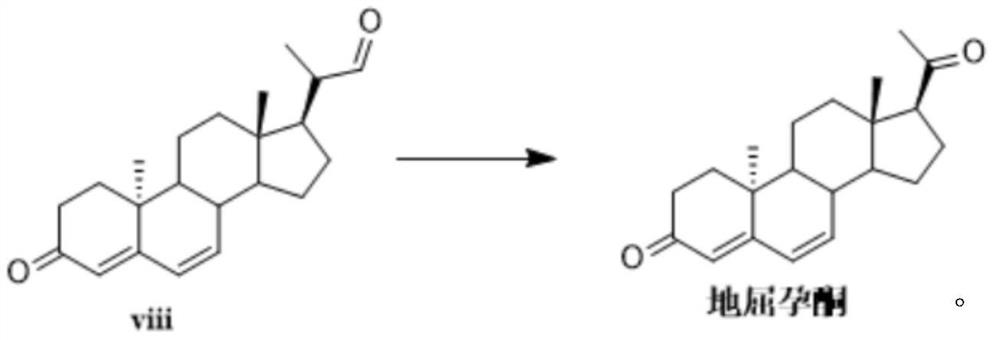
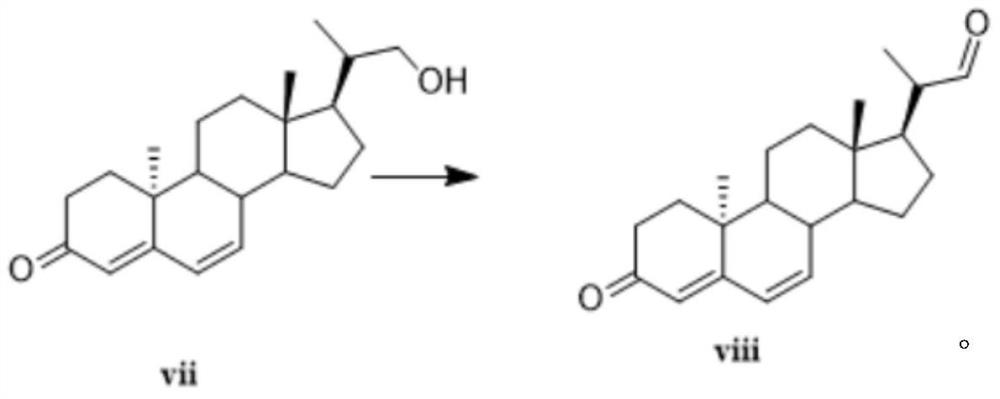
Novel method for synthesizing didrogesterone, and compound
A synthesis method and compound technology, which is applied in the production of steroids, organic chemistry, bulk chemicals, etc., can solve the problems of synthetic dydrogesterone, etc., and achieve the effect of easy-to-obtain raw materials and simple and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] N 2 Under protection, bisnaniline BA (500 g, 1.513 mol) and triethylamine (229.6 g, 2.269 mol) were dissolved in 3LDCM, and the temperature was lowered to 0-5°C. Under stirring, chloroacetyl (130.6 g, 1.664 mol) was added dropwise to the above reaction solution, and the temperature was controlled not to exceed 10°C. After the dropwise addition, stir for 1 h, and add 1 L of ice water to quench. After static liquid separation, the organic phase was washed with water, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 541 g of white solid compound i, HPLC=97%, yield 96%.
Embodiment 2
[0057] Dissolve BA (300 g, 0.908 mol) and 4-dimethylaminopyridine (166.3 g, 1.361 mol) in 2.4 LDCM and lower the temperature to 5-10°C. N 2Under protection, acetic anhydride (102.1 g, 0.953 mol) was added dropwise to the above reaction solution, and the temperature was controlled not to exceed 10°C. After the dropwise addition, stir for 3 h, then add 500 ml of ice water to quench. Static liquid separation, organic phase with saturated NaHCO 3 Washed with aqueous solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 331 g of white solid compound i, HPLC=99%, yield 97.8%.
[0058] The obtained compound i: 1 HNMR (400MHz, CDCl 3 )δ5.73(s, 1H), δ4.06-4.09(q, 1H), 3.75-3.79(q, 1H), 2.24-2.45(m, 4H), 2.05(s, 3H), 2.0-2.04( m, 2H), 1.34-1.86(m, 9H), 1.12-1.24(m, 6H), 0.91-1.05(m, 6H), 0.74(s, 3H)
[0059] Synthesis of compound ii series
Embodiment 3
[0061] Under stirring, add 4L of anhydrous toluene, 389g of compound i (1.044mol), 866g of ethylene glycol and 10g of p-toluenesulfonic acid monohydrate into a 10L reactor with a reflux separator, heat to 120°C to reflux and separate water . After stirring for 3 h, TLC detected that the starting material disappeared. Cool down to room temperature, add 2 L of water and stir for 30 min, then separate the layers, and wash the organic phase twice with 1 L of water. The organic phase was concentrated under reduced pressure to obtain the crude compound ii. The obtained crude product was added into 500ml of methanol, refluxed, lowered in temperature, slurried and filtered to obtain 343g of white solid compound ii, HPLC=96%, yield 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com