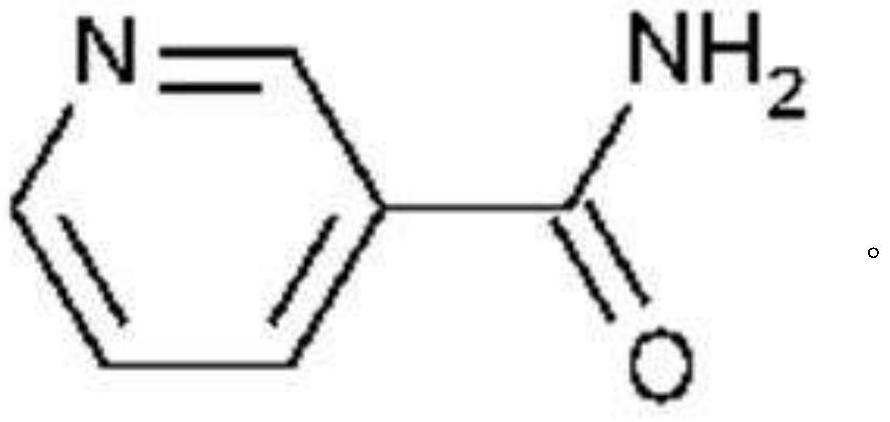
Nicotinamide for inducing antimicrobial peptide production
A technology of nicotinamide and its application, which is applied in the direction of antibacterial drugs, drug combination, drug delivery, etc., and can solve the problem of impaired ability to clear Staphylococcus aureus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 6
[0035] Examples 1 to 6: Demonstration that nicotinamide induces LL-37 gene expression but does not alter psoriasin expression Example of
[0036] The following samples as shown in Table 1 were prepared in order to measure their effects on psoriasis expression and expressed psoriasis AMP (LL-37).
[0037] Table 1
[0038] Example sample 1 Controls for LL-37 gene expression 2 Positive control LPS (25↘g / ml) for LL-37 gene expression 3 Nicotinamide (1.22mg / ml) for LL-37 gene expression 4 Controls for psoriasis gene expression 5 Positive control LPS (25↘g / ml) for psoriasis gene expression 6 Niacinamide (1.22mg / ml) for psoriasis gene expression
[0039] .
[0040] Materials and procedures used to measure the expression of each gene are given below.
[0041] Material
[0042] Normal Human Neonatal Epidermal Primary Keratinocytes (NHEK), Keratinocyte Growth Medium (KGM) and Growth Supplements, Antibiotics (Penicillin and Stre...
Embodiment 7 to 12
[0055] Examples 7 to 12: In vivo experiments using skin lotions containing niacinamide to demonstrate the Secretion of dermatoxin and LL-37 To confirm the findings from in vitro experiments, the effects of nicotinamide were tested in vivo on healthy human volunteers. Volunteers were asked to apply lotion with and without niacinamide (3%) twice daily on their forearms. After 7 days of application, spots were extracted in PBS and ELISA was performed to detect psoriasis and LL-37AMP.
[0056] In vivo study procedure
[0057] E. coli culture preparation
[0058] A glycerol stock of E. coli (10536) was inoculated in 30 ml TSB medium and the culture was incubated overnight at 37°C in a shaking incubator. Overnight cultures were subcultured on TSA slants and incubated overnight at 37°C before storing the slants at 4°C (slants were freshly prepared once 15 days old).
[0059] E. coli cultures from slants were subcultured on TSA plates and incubated overnight at 37°C. Plate...
Embodiment 13
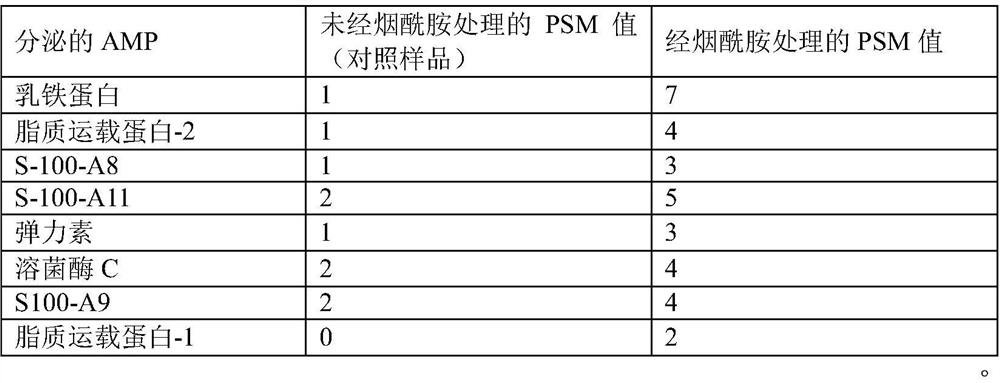
[0088] Example 13: Experiments to determine the type and amount of antimicrobial peptide (AMP) secreted by nicotinamide induction The following procedure was used to determine AMP induced by nicotinamide on keratinocytes.
[0089] 1. Seed primary keratinocytes in a 12-well plate with 1 ml KGM complete medium. (60,000 cells / well seeded at passage 4).
[0090] 2. Place the plate in CO 2 Incubate for 48 hours in an incubator.
[0091] 3. After 48 hours of incubation, cells were treated in duplicate wells with and without nicotinamide at a concentration of 1 mg / ml (fresh medium was used for treatment).
[0092] 4. After 72 hours of incubation, the cell culture supernatants were transferred to 1.5 ml microcentrifuge tubes respectively.
[0093] 5. The sample was then passed through a 20 kDa cut-off filter to remove high molecular weight proteins.
[0094] 6. Transfer the eluted sample containing <20kDa protein to a 15ml sterile centrifuge tube.
[0095] 7. Subsequently, add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com