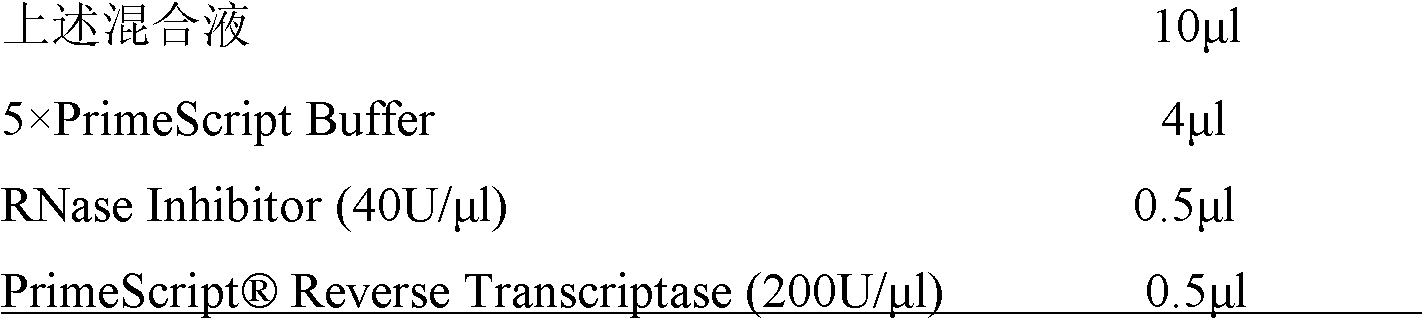Antimicrobial peptide Hainanenin-5 of Amolops hainanensis, gene of antimicrobial peptide Hainanenin-5 of Amolops hainanensis, separation and purification method and chemical synthesis method for antimicrobial peptide Hainanenin-5 of Amolops hainanensis and application of gene of antimicrobial peptide Hainanenin-5 of Amolops hainanensis
A hainanenin-5, antimicrobial peptide technology, applied in the field of biomedicine, to achieve broad-spectrum antibacterial activity, high stability, and broad-spectrum drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]Isolation and purification of Hainanenin-5: adult Hainannensis frogs (A.hainanensis) (n=5) in good health were selected, and their backs were rinsed with ultrapure water. The skin secretions were collected by electric stimulation method, and the skin on the back of the frog was electrically stimulated with an electric shocker, the voltage was I0V, and the electric shock time was 3ms. After the stimulation, the skin secretions were washed with 0.9% normal saline into a clean container, and centrifuged at 12000rpm After 20 min, the supernatant was discarded, freeze-dried and stored. Store at -20°C for later use.
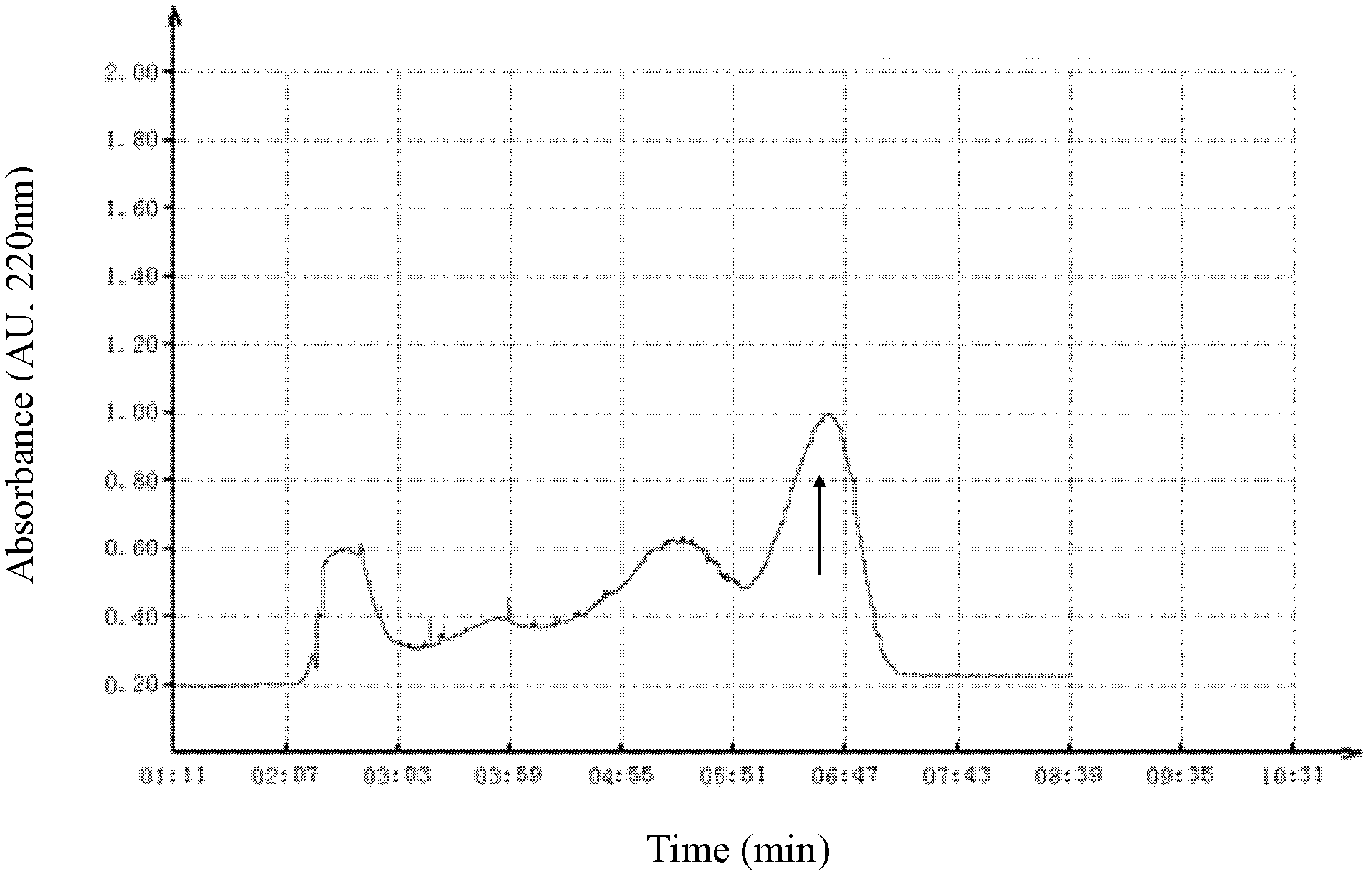
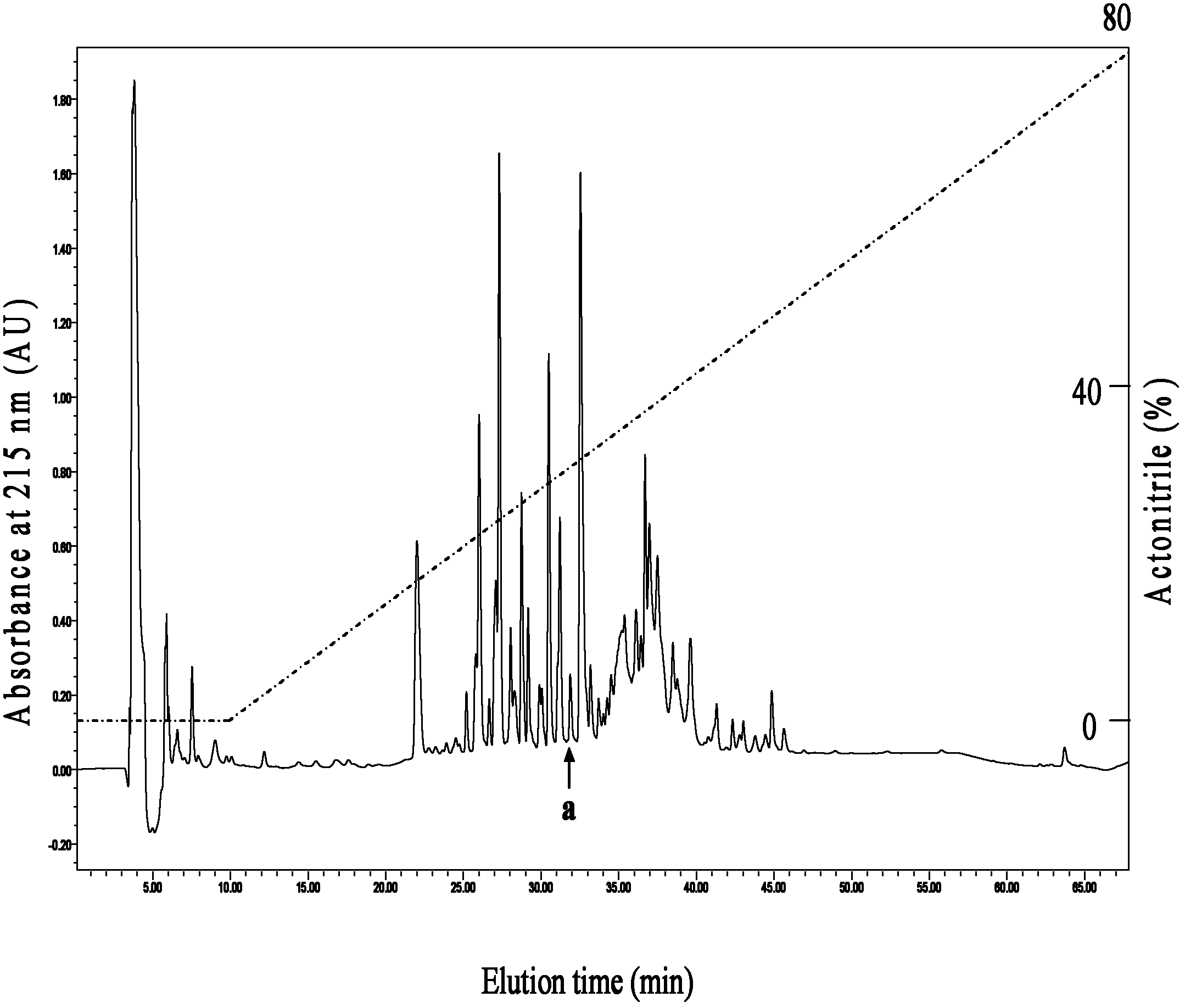
[0025] The first step Sephadex G-50 gel filtration chromatography: 0.9gA.hainanensis back skin secretion freeze-dried powder with 10ml0.1M phosphate (Na 2 HPO 4 -NaH 2 PO 4 , pH6.0) buffer solution, centrifuged at 12000rpm for 10min, and the supernatant was loaded onto a balanced Sephadex G-50 gel exclusion chromatography column (1.6cmx90cm, Amersham Bioscien...
Embodiment 2
[0029] Cloning and gene sequencing of antimicrobial peptide Hainanenin-5 precursor gene, including:
[0030] AxyPrepTM Multisource Total RNA Miniprep Kit was used to extract total RNA from the skin of Amolops hainanensis, and the Creator TM SMART TM The cDNA library construction kit was used to construct the skin cDNA library of Amolops hainanensis. Reverse Transcriptase reverse transcription synthesizes the first strand of cDNA, the primers are:
[0031] Forward SMARTer V Oligonucleotide: 5′-AAGCAGTGGTATCAACGCAGAGTACXXXXX-3′(X=undisclosed base in the proprietary SMARTer oligo sequence)
[0032] Reverse 3'In-Fusion SMARTer CDS Primer:
[0033] 5'-CGGGGTACGATGAGACACCATTTTTTTTTTTTTTTTTTTTTTVN-3' (N = A, C, G, or T; V = A, G, or C).
[0034] Use Advantage DNA Polymerase to synthesize the second strand, the primer is: forward 5'-primer:
[0035] 5'-AAGCAGTGGTATCAACGCAGAGT-3'. The reverse primer is also 3'In-Fusion SMARTer CDS Primer.
[0036] A specific forward primer (P...
Embodiment 3
[0083] The chemical synthesis method of antimicrobial peptide Hainanenin-5:
[0084] 1. According to the deduced amino acid sequence of the mature peptide Hainanenin-5, its full sequence was synthesized with an automatic peptide synthesizer (Applied Biosystems), and desalted and purified by HPLC reverse-phase column chromatography.
[0085] 2. The molecular weight was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com