Separation and purification method of tacrolimus
A tacrolimus, separation and purification technology, applied in the field of biopharmaceuticals, can solve problems such as high cost and difficult industrial production, and achieve the effect of improving purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The separation and purification method of tacrolimus in this embodiment comprises the following steps:
[0063] 1) Use a ceramic membrane to filter 30L tacrolimus fermentation broth (the content of tacrolimus in the fermentation broth is 1.054mg / mL, as determined by HPLC, the fermentation broth contains 31.62g of tacrolimus, and the tacrolimus in the fermentation broth The purity is 63.45%), and use 90L of water to wash the filter cake to obtain ceramic membrane dope 10L. Wherein, the pore diameter of the ceramic membrane is 50 nm.
[0064] 2) Add ethanol to the ceramic membrane dope until the volume concentration of ethanol in the dope is 30%, then add 3 L of Huazhen HZ806 resin, stir for 2 hours and then filter, rinse the filtered resin with a small amount of water, and wash the 3 L of Huazhen Pack the column with Zhen HZ806 resin to get the first resin column, then use 6L Huazhen HZ806 resin to pack the column to get the second resin column, and then use 15L Huazhen...
Embodiment 2
[0071] The separation and purification method of tacrolimus in this example is basically the same as that in Example 1, except that:
[0072] In step 1), the tacrolimus content in the tacrolimus fermentation broth used was 1.07 mg / mL, the fermentation broth contained 32.1 g of tacrolimus, and the tacrolimus purity in the fermentation broth was 64.12%.
[0073] In step 2), ethanol with a volume concentration of 50% is used as the eluent to elute the series resin column.
[0074] Finally, 22.1 g of tacrolimus was obtained with a purity of 99.09% and a total yield of 68.56%.
Embodiment 3
[0076] The separation and purification method of tacrolimus in this example is basically the same as that in Example 1, except that:
[0077] In step 1), the content of tacrolimus in the tacrolimus fermentation broth used was 1.01 mg / mL, the fermentation broth contained 30.3 g of tacrolimus, and the purity of tacrolimus in the fermentation broth was 63.77%.
[0078] In step 2), ethanol with a volume concentration of 45% is used as the eluent to elute the series resin column.
[0079] Finally, 20.75 g of tacrolimus was obtained with a purity of 99.59% and a total yield of 68.48%.
[0080] In this example, after obtaining the crude product of tacrolimus chromatography, the resin in the first resin column was regenerated with absolute ethanol, the regenerated solution appeared red, and the regenerated resin returned to white.
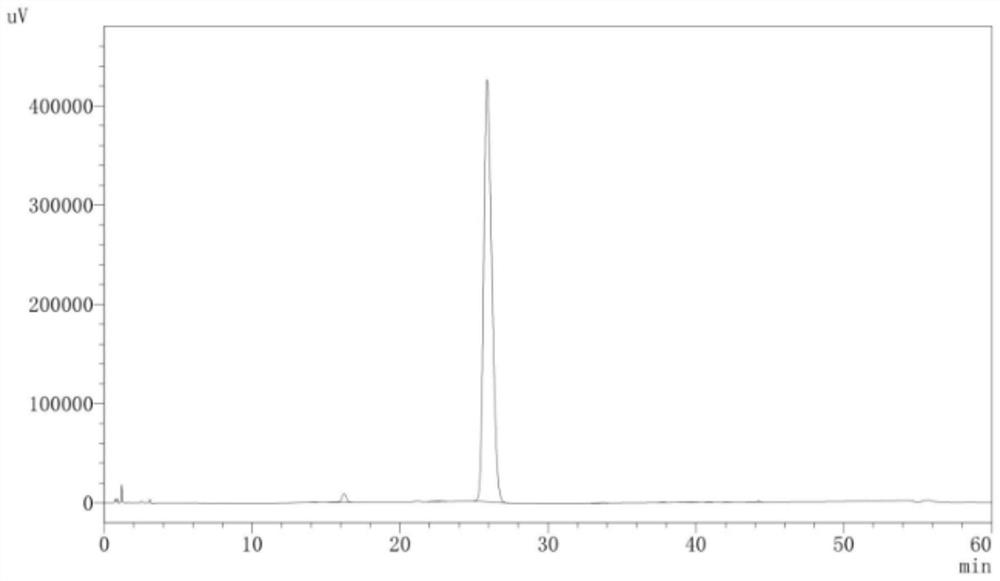
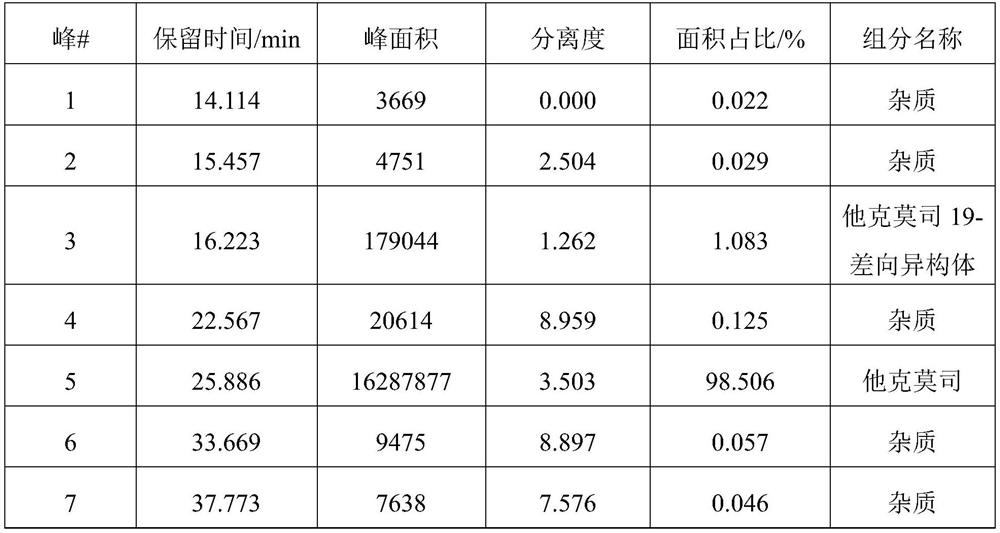
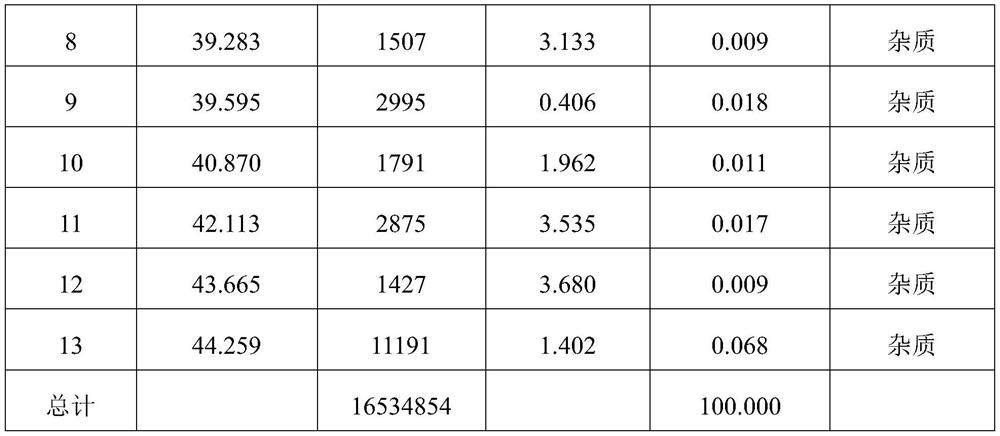
[0081] figure 1 It is the pure product liquid phase chromatogram of tacrolimus of embodiment 3, to figure 1 The relevant information in Table 1 can be ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com