Single-domain heavy-chain antibody, encoding gene, preparation method and use thereof and pharmaceutical composition
A single-domain heavy chain antibody and composition technology, applied in the biological field, can solve the problems of difficulty and lack of MERS vaccine development, and achieve good neutralizing activity, strong reactivity, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
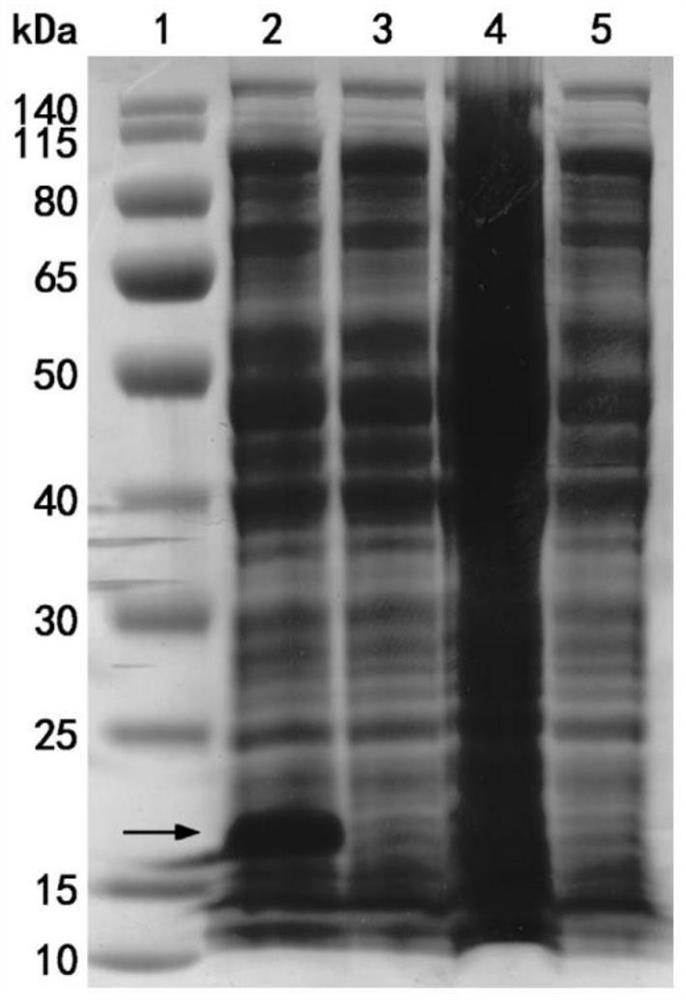
[0080] This example is used to illustrate the expression and identification of the single domain heavy chain antibody Nano-Anti-MRBD.
[0081] (1) Expression vector construction
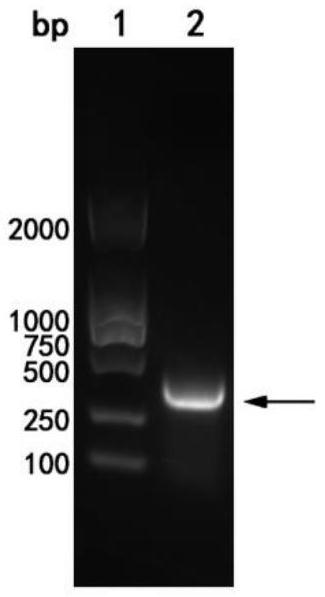
[0082] The inventor designed the nucleotide sequence shown in SEQ ID NO: 2 as the coding gene of the single domain heavy chain antibody Nano-Anti-MRBD (amino acid sequence shown in SEQ ID NO: 1), wherein the 5' end is the first -6 is the recognition site of BamH I, the 7th-363rd (i.e. the nucleotide sequence shown in SEQ ID NO:3) is the Nano-Anti-MRBD coding sequence, and the 364th-366th is a stop codon, Positions 367-372 are the recognition sites of Xba I.
[0083] The coding gene of the single domain heavy chain antibody Nano-Anti-MRBD and the pCold I vector (purchased from TAKARA Company, the brand is D3361, which The expressed protein carries a Poly-His tag (the amino acid sequence of the tag is shown in SEQ ID NO: 6) for double enzyme digestion, and the digested product is ligated to obtain th...
Embodiment 2
[0108] This example is used to illustrate the binding properties of the single domain heavy chain antibody Nano-Anti-MRBD to MERS-CoV.
[0109] The indirect ELISA method was used to detect the reactivity of Nano-Anti-MRBD and MERS-CoV RBD, the specific method is as follows:
[0110] Use the MERS-CoV RBD-Fc protein (the fusion protein of the RBD functional region of the MERS-CoV S protein and the human IgG Fc fragment, the amino acid sequence of which is shown in SEQ ID NO: 4) to coat a 96-well microtiter plate at a coating concentration of 1 μg / mL, 50 μL per well. The concentrated Nano-Anti-MRBD protein sample obtained in Example 1 was prepared into protein solutions with different concentrations as the primary antibody in the experimental group using blocking solution (PBS solution containing 3% by weight BSA), and HRP-His mouse monoclonal antibody (purchased Zikangwei Century Company (brand name: CW0285M) was used as the secondary antibody. In the control group, SARS-CoV a...
Embodiment 3
[0113] This example is used to illustrate the neutralizing activity of single domain heavy chain antibody Nano-Anti-MRBD to MERS-CoV pseudovirus.
[0114] The Huh-7 cells used in this example were purchased from ATCC, and the preparation method of the MERS-CoV pseudovirus (containing firefly luciferase reporter gene) used was referred to: A safe and convenient pseudovirus-basedinhibition assay to detect neutralizing antibodies and screen for Viral entry inhibitors against the novel human coronavirus MERS-CoV. Zhao G, Du L, Ma C, et al. Virol J. 2013;10:266. doi:10.1186 / 1743-422X-10-266.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com