PD-L1 targeting immunotoxin as well as preparation method and application thereof
A PD-L1, immunotoxin technology, applied in the field of immunotoxin targeting PD-L1 and its preparation, can solve problems such as disease progression or recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
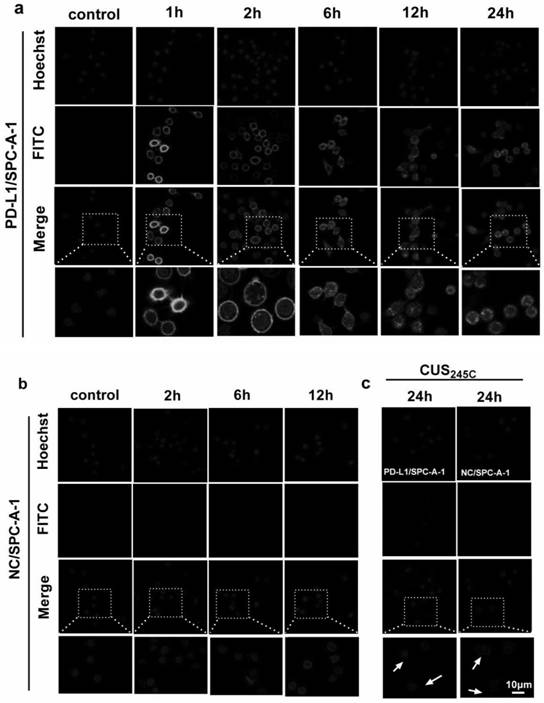
[0015] Example 1: Fluorescence-labeled Durvalumab tracks binding to PD-L1 and its internalization process
[0016] Method: The human lung cancer cell line PD-L1 / SPC-A-1 transfected with PD-L1 and the parental cell line SPC-A-1 cell line (named NC / SPC-A-1 in this specification) in logarithmic growth phase cell line). After digestion and counting, dilute to 5×10 with RPMI1640 complete medium containing 10% FBS 4 cells / ml, add to confocal special glass culture dish, add 1ml of cells per well, place at 37°C, 5% CO 2 Cultivate in an incubator for 24 hours, suck away the cell culture medium in the culture dish, and use RPMI 1640 to put the CUS 245C -FITC and Durvalumab-FITC were diluted to 5ng / ml to treat the cells. After the cells were treated for 1h, 2h, 6h, 12h and 24h, the liquid in the well was discarded, and the internalization was terminated with pre-cooled PBS. Discard the PBS in the well, and wash with pre-cooled PBS for 3 times, dilute Hoechst into 1× working solution a...
Embodiment 2
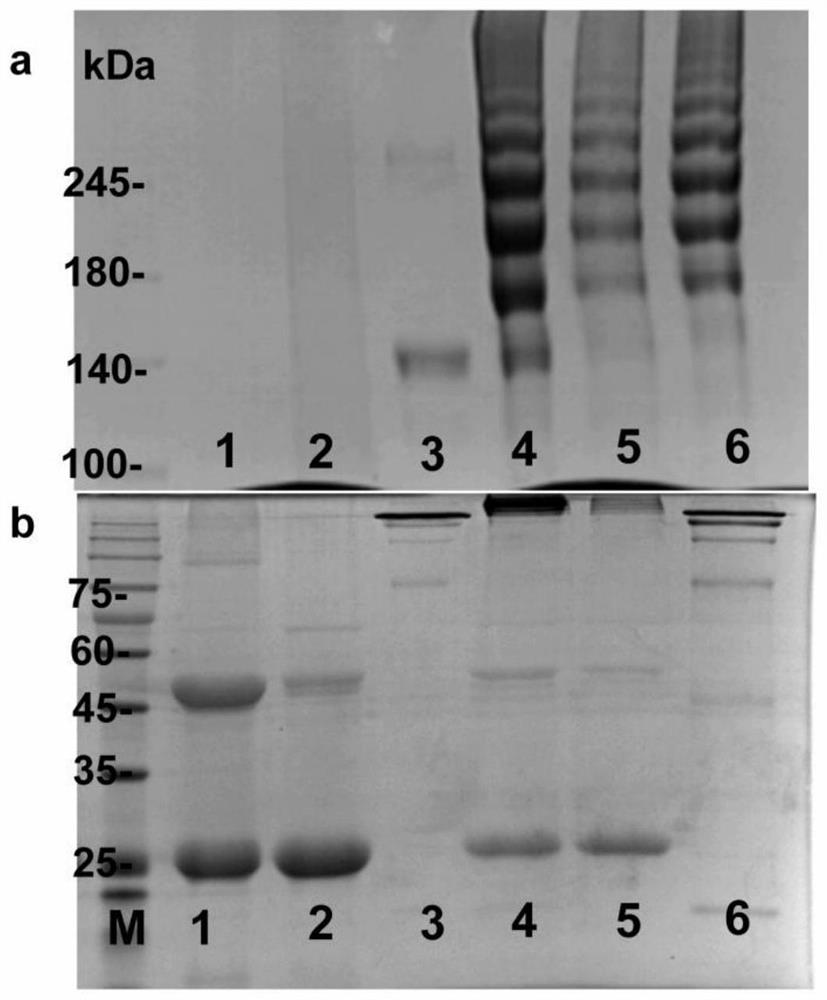
[0019] Example 2: Durvalumab and pumpkin protein mutant CUS 245C Coupling to prepare immunotoxin D-CUS 245C
[0020] Method: Take 2ml of Durvalumab (5mg / ml), dialyze with a dialysis tube with a molecular weight cut-off of 8-10kDa, and change the PBS dialysate every 3 hours (the amount of PBS is 1L each time), a total of 5 times; after dialysis, 16.45μl 20mmol / L SPDP solution (dissolved in DMSO), stirred at room temperature for 30 min, and the reaction product (D-PDP) was dialyzed with the above buffer solution overnight to remove excess SPDP. Take another 3ml CUS 245C (a total of 7.45mg), add an appropriate amount of dithiothreitol (DTT) dissolved in 0.01mol / L NaAc, so that the final concentration of DTT is 0.3mol / L, stir at room temperature for 30min, and perform dialysis with a 8-10kDa dialysis column. The method is the same as above, remove excess DTT; mix the above-mentioned Durvalumab after dialysis with CUS 245CMix in a small beaker, stir and react at 23°C for 18 hou...
Embodiment 3
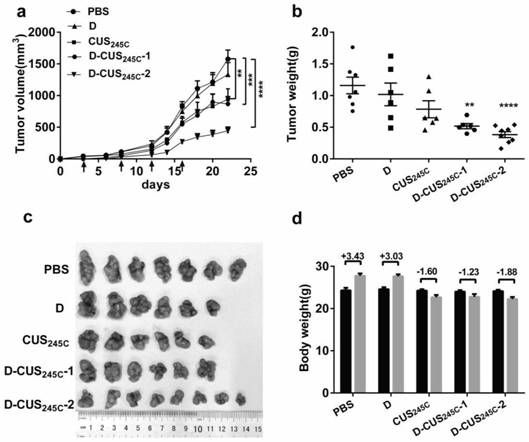
[0023] Example 3: Immunotoxin D-CUS 245C In vitro antitumor activity assay
[0024] Methods: The PD-L1 overexpressing human breast cancer cell line MDA-MB-231 cells in the logarithmic growth phase and the PD-L1 transfected human lung cancer cell lines PD-L1 / SPC-A-1 and PD-L1 low The expressed lung cancer cell line NC / SPC-A-1 was inoculated on a 96-well culture plate, and the inoculation volume in each well was 4000 / 100 μL. After culturing for 24 h, the experimental group was added different concentrations of immunotoxin (D-CUS 245C ), squash mutant (CUS 245C ), Durvalumab (D) and the mixture of pumpkin protein and Durvalumab of corresponding concentration (D+CUS 245C ) 100 μL, and three replicate wells were made for each concentration; 100 μL of nutrient solution was added to the negative control group, and 200 μL of nutrient solution was added to the blank control wells for zero adjustment of the instrument. After 72 h or 120 h of action, discard the supernatant, add 100 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com