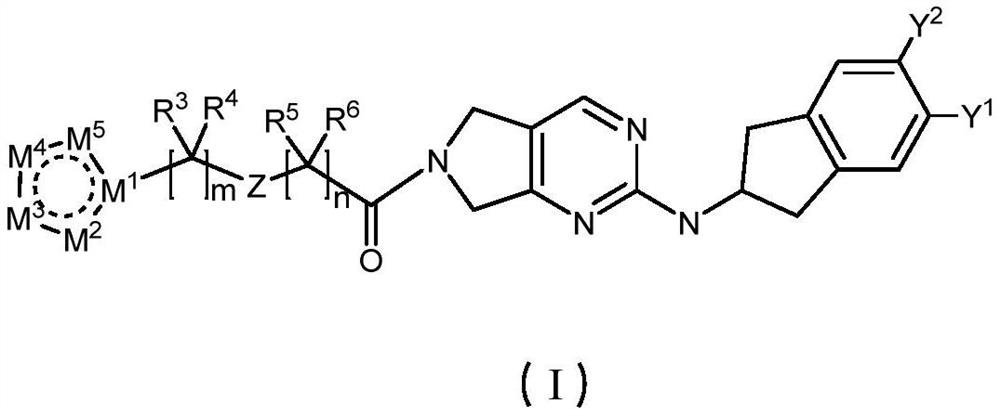
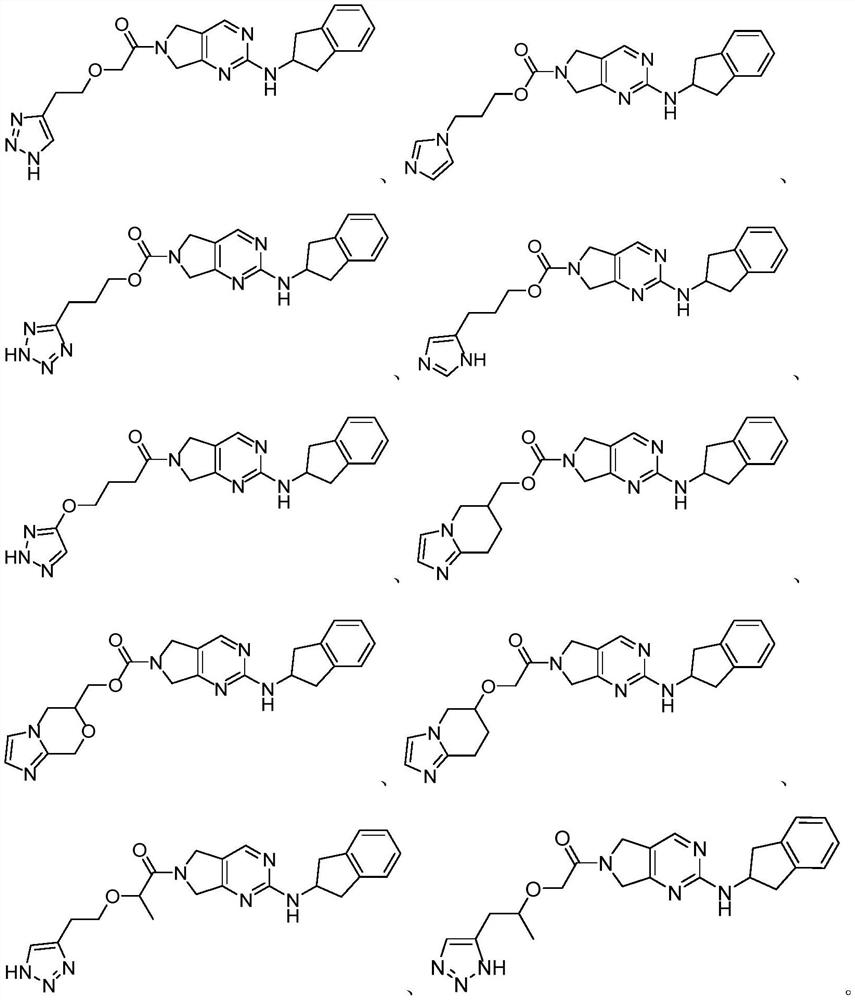
Pyrrolopyrimidine derivative and application thereof
A compound and hydrate technology, applied in the preparation of drugs, pyrrolopyrimidine derivatives and its preparation, the field of pyrrolopyrimidine derivatives, can solve the problem that patients with severe specific pulmonary fibrosis cannot benefit and cannot improve the quality of life of patients , can not reverse pulmonary fibrosis and other problems, and achieve significant heart safety advantages, excellent liver metabolism stability, and high intestinal absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
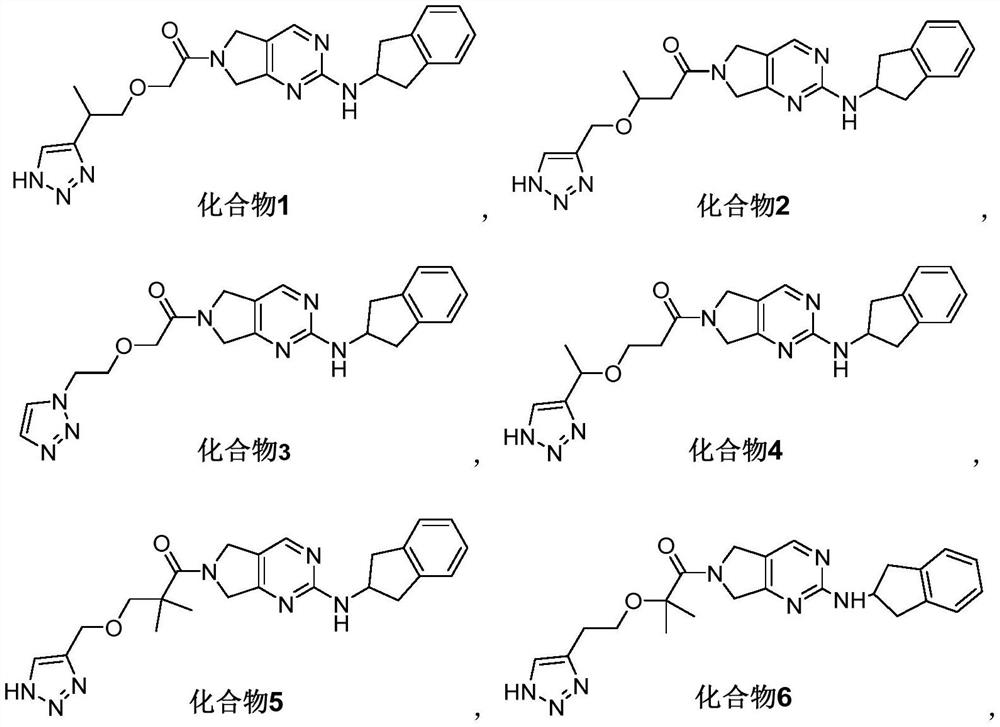
[0145] Embodiment 1: the synthesis of compound 1, compound 1S and compound 1R
[0146] 2-(2-(1H-1,2,3-triazol-4-yl)propoxy)-1-(2-((2,3-dihydro-1H-inden-2-yl)amino) -5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidin-6-yl)ethan-1-one (compound 1)
[0147] (S)-2-(2-(1H-1,2,3-triazol-4-yl)propoxy)-1-(2-((2,3-dihydro-1H-indene-2- Base) amino)-5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidin-6-yl)ethan-1-one (compound 1S)
[0148] (R)-2-(2-(1H-1,2,3-triazol-4-yl)propoxy)-1-(2-((2,3-dihydro-1H-indene-2- Base) amino)-5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidin-6-yl)ethan-1-one (compound 1R)
[0149] The synthetic routes of target compound 1, compound 1S and compound 1R are as follows:
[0150]
[0151] The first step: the synthesis of 3-((tert-butyldimethylsilyl)oxy)-2-methylpropan-1-ol (compound 1B)
[0152] To a solution of 2-methylpropane-1,3-diol (compound 1A) (15 g, 166 mmol) in dichloromethane (200 mL) was added tert-butyldimethylsilyl chloride (25.09 g, 166 mmol) and triethylamine (33.7g, 333mm...
Embodiment 2
[0179] Embodiment 2: the synthesis of compound 2
[0180] 3-((1H-1,2,3-triazol-4-yl)methoxy)-1-(2-((2,3-dihydro-1H-inden-2-yl)amino)-5 ,7-dihydro-6H-pyrrole[3,4-d]pyrimidin-6-yl)butan-1-one (Compound 2)
[0181] The synthetic route of target compound 2 is as follows:
[0182]
[0183] The first step: (E)-1-(2-((2,3-dihydro-1H-inden-2-yl)amino)-5,7-dihydro-6H-pyrrole[3,4-d] Synthesis of pyrimidin-6-yl)but-2-en-1-one (compound 2B)
[0184] Crotonic acid (compound 2A) (142mg, 1.646mmol), N-(2,3-dihydro-1H-inden-2-yl)-6,7-dihydro-5H-pyrrole[3,4-d] Pyrimidin-2-amine dihydrochloride (535mg, 1.646mmol), N,N-diisopropylethylamine (2.127g, 16.46mmol) was dissolved in N,N-dimethylformamide (5mL), and the reaction The solution was stirred and cooled to about 0°C, and 2,4,6-tripropyl-1,3,5,2,4,6-trioxytriphosphoric acid-2,4,6-trioxide (1.257g, 1.975mmol, 50% N,N-dimethylformamide solution), the dropwise addition was completed, and the reaction was carried out at room temperature f...
Embodiment 3
[0193] Embodiment 3: the synthesis of compound 3
[0194] 2-(2-(1H-1,2,3-triazol-1-yl)ethoxy)-1-(2-((2,3-dihydro-1H-inden-2-yl)amino) -5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidin-6-yl)ethan-1-one (compound 3)
[0195] The synthetic route of target compound 3 is as follows:
[0196]
[0197] The first step: 2-chloro-1-(2-((2,3-dihydro-1H-inden-2-yl)amino)-5,7-dihydro-6H-pyrrolo[3,4-d Synthesis of ]pyrimidin-6-yl)ethan-1-one (compound 3B)
[0198] At room temperature, triethylamine (0.289ml, 2.072mmol) was added dropwise into the stirring N-(2,3-dihydro-1H-inden-2-yl)-6,7-dihydro-5H-pyrrolo[ 3,4-d] Pyrimidin-2-amine dihydrochloride (200 mg, 0.615 mmol) in dichloromethane (5 mL). After 5 minutes, chloroacetyl chloride (81 mg, 0.713 mmol) was added dropwise into the above reaction solution at 0° C., and stirred overnight at room temperature. After monitoring the reaction, add distilled water (20mL) for dilution, extract with ethyl acetate (30mL×3), combine the organic phases, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com