Method for establishing kidney organoids through stem cell induced differentiation
A technology for inducing differentiation and organoids, applied in the field of cell engineering, can solve the problems of low differentiation efficiency and unstable differentiation, and achieve the effect of high differentiation efficiency, stable differentiation, and stable establishment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Induced differentiation of stem cells to establish kidney organoids
[0043] 1.1 Establishment of 2D kidney organoids
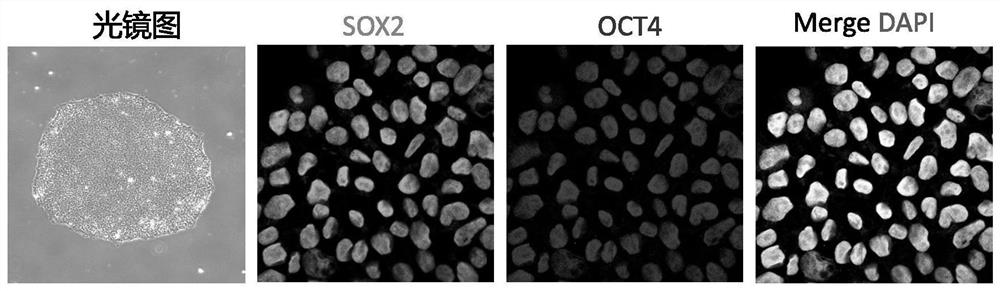
[0044] 1. Human induced pluripotent stem cells were cultured in mTesR medium at 37°C with 5% CO 2 In the incubator, change the medium every day to keep the cell growth in good condition (such as figure 1 As shown in the light microscope), immunofluorescence staining showed that the markers of the pluripotent state were well expressed (such as figure 1 Fluorescent staining is shown).
[0045]2. When the cell density reaches 70%-80%, digest with Accutase to prepare a single cell suspension, add 10μM Rock inhibitor according to 1.5×10 4 / cm 2 Seed the cells at the cell density. at 5% CO 2 After culturing in an incubator at 37°C for 24 hours, change the medium and continue culturing for 48 hours.
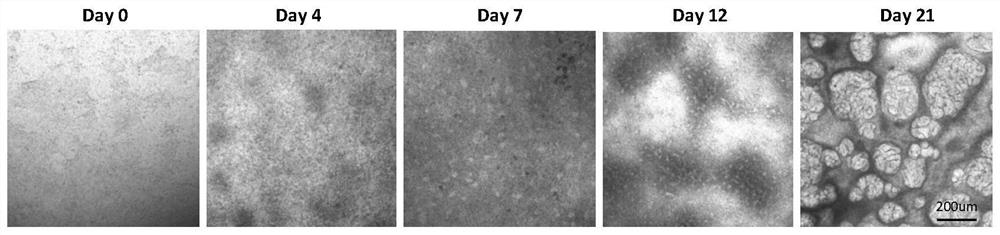
[0046] 3. When the cells grow to 50% density (differentiation day 0), add wnt signaling pathway activator CHIR-99021 (10 μM) and TGF-signaling ...
Embodiment 2
[0057] Example 2 compares the effects of different reagents on stem cell induced differentiation
[0058] The present invention compares the influence of different reagents on the differentiation of human induced pluripotent stem cells, and the specific experimental conditions are shown in the following table:
[0059]
[0060]
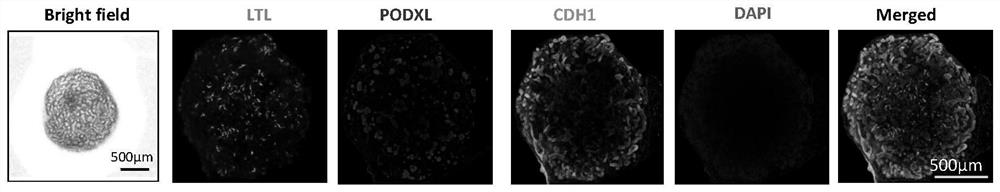
[0061] The differentiation results of the 24 differentiation methods in the above table are as follows: Figure 4 As shown, only the conditions 16, 17, and 18 were used, and the differentiation of human induced pluripotent stem cells was successful. Among them, compared with differentiation condition 16 and 17 and 18, the structure obtained by differentiation under condition 16 was clearer and more mature.
Embodiment 3
[0062] Example 3 Comparison of Induced and Differentiated Results of Stem Cells
[0063] Experimental group: using the method of Example 1 of the present invention to induce differentiated stem cells;
[0064] Control group: for differentiation day0-day4, add CHIR-99021 10μM to the differentiation medium; day4-day7, add ActivinA 10ng / ml to the differentiation medium; day7-day9, add FGF9 10ng / m to the differentiation medium; From day9 to day11, FGF9 10ng / ml and CHIR-99021 3μM were added to the differentiation medium; from day9 to day20, FGF9 10ng / ml was added to the differentiation medium.
[0065] Using the differentiation method of the control group, the differentiation results are unstable, and differentiation failure (without any structure) often occurs in the late stage of differentiation or occasionally some incomplete results appear (such as Figure 5 shown on the left), Figure 5 The figure on the right shows the results obtained by the method of the experimental grou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com