Culture medium of endometrial organ and culture method
A technology of endometrium and culture method, which is applied in the field of culture medium and culture of endometrium organoids, can solve the problem of lack of endometrial organoid research and reports, test procedures, operation steps, culture conditions, and culture medium formulations. Too many reports and other issues, to achieve the effect of rapid expansion, wide application range, and fast growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
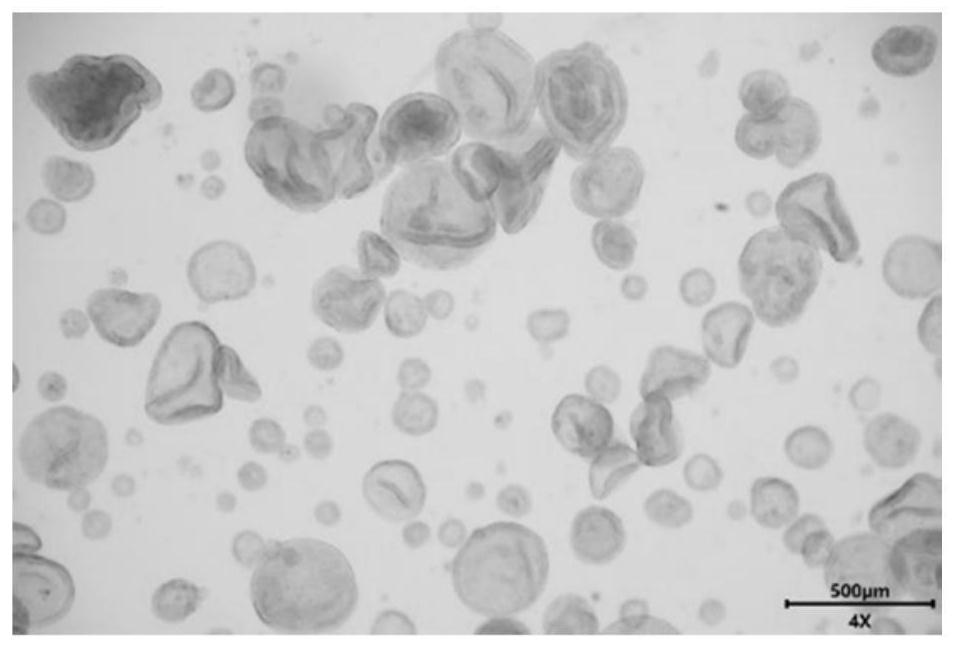
[0032] This embodiment provides an endometrial cancer organoid culture medium and its culture method. The endometrial cancer organoid culture medium is composed of: 80ng / ml EGF, 150ng / ml Noggin, 250ng / ml R- spondin 1, 150ng / ml Wnt3a, 25ng / ml FGF9, 60ng / ml KIAA1199, 20ng / ml sox2, 80nM isoflavones, 500nM CHIR99021, 8μM A83-01, 8μM RKI-1477, 100ug / ml of primary cell antibiotics. The methods for culturing endometrial cancer organoids are as follows:
[0033] 1) Sample washing: transfer the obtained endometrial cancer specimen into a centrifuge tube, then shake and wash with sterile normal saline for 30 seconds, remove the supernatant, and re-add sterile normal saline for washing. Repeated washing 3 times according to the above method to remove impurities on the tissue surface.
[0034] 2) Sample shearing: In a biological safety cabinet, transfer the sample to a 6cm culture dish, use sterilized surgical scissors on ice to cut the tissue to a size of 1-5mm 3 .
[0035] 3) Sampl...
Embodiment 2
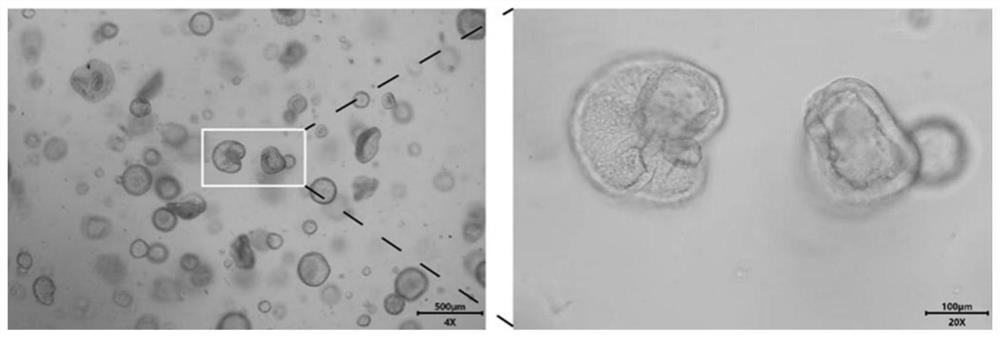
[0041] This embodiment provides an endometrial cancer organoid culture medium and its culture method. The endometrial cancer organoid culture medium is composed of: 20ng / ml EGF, 400ng / ml Noggin, 80ng / ml R- spondin 1, 400ng / ml Wnt3a, 50ng / ml FGF9, 100ng / ml KIAA1199, 80ng / ml sox2, 20nM isoflavones, 1000nM CHIR99021, 20μM A83-01, 18μM RKI-1477, 50ug / ml of primary cell antibiotics. The method for culturing endometrial cancer organoids is the same as in Example 1. After 6 days of culture, endometrial cancer organoids can be obtained, and the tissue morphology and structure are observed under an ordinary optical microscope as figure 2 shown.
Embodiment 3
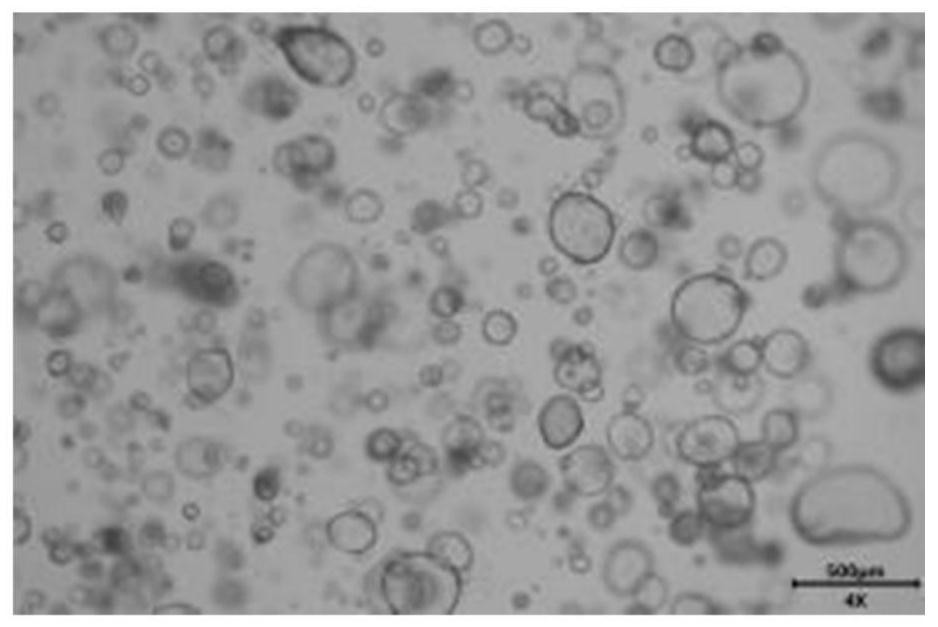
[0043] This embodiment provides an endometrial organoid culture medium and a culture method. The endometrial organoid culture medium is composed of: 50ng / ml of EGF, 100ng / ml of Noggin, and 500ng / ml of R-spondin 1 according to the final concentration , 100ng / ml Wnt3a, 50ng / ml FGF9, 30ng / ml KIAA1199, 50ng / ml sox2, 15nM isoflavones, 200nM CHIR99021, 15μM A83-01, 10μM RKI-1477, 100ug / ml Pro generation of cellular antibiotics. The endometrial organoid culture method is:
[0044]1) Sample washing: Transfer the obtained endometrium sample into a centrifuge tube, then shake and wash with sterile normal saline for 30 seconds, remove the supernatant, and add sterile normal saline again for washing. Repeated washing 3 times according to the above method to remove impurities on the tissue surface.
[0045] 2) Sample shearing: In a biological safety cabinet, transfer the sample to a 6cm culture dish, use sterilized surgical scissors on ice to cut the tissue to a size of 1-5mm 3 .
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com