A kind of antidrug antibody combined with coronavirus bispecific antibody and preparation method and application thereof
A bispecific antibody, coronavirus technology, applied in the field of biomedicine, can solve problems such as loss, drug resistance escape, and vaccine protection decline, and achieve the effect of high binding affinity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Example 1 Animal Immunization
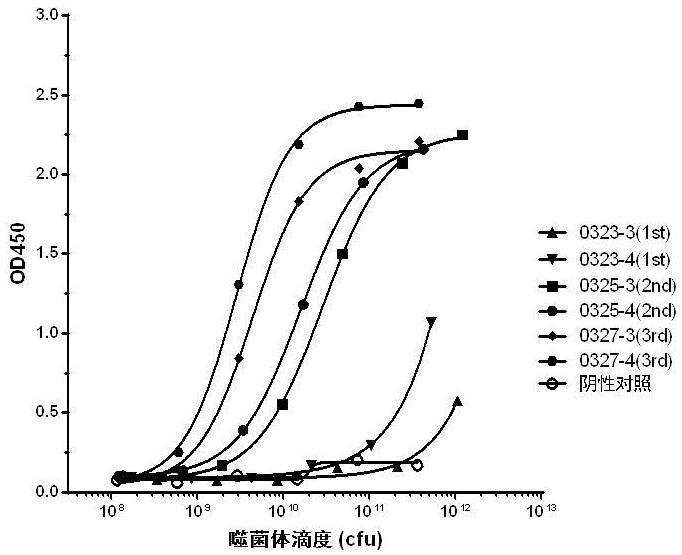
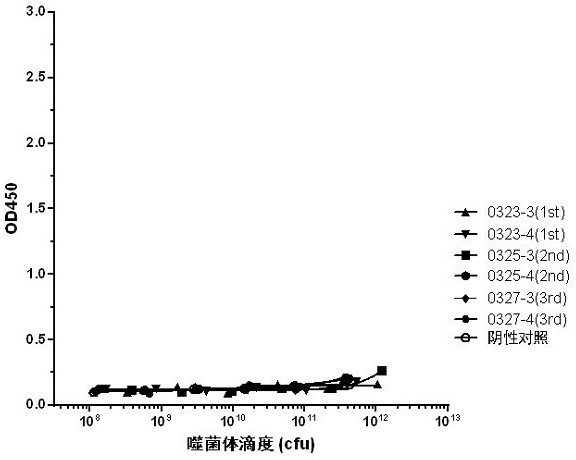
[0104] The immunogenic protein used for antibody preparation is BsAb17 bispecific antibody, and the production and preparation process of BsAb17 bispecific antibody refers to patent application CN202011300574.2. The BsAb17 antibody was used as an immunogen to immunize Balb / C mice (Shanghai Lingchang Biotechnology Co., Ltd.). A total of 4 mice were immunized. The method of subcutaneous multi-point immunization was adopted, 50 μg subcutaneously each time, and immunized every two weeks. Once, a total of four immunizations. The titer of immunization was determined by ELISA after four immunizations. The test results showed that the immune titer reached 1:1458000, and the titer test results are shown in Table 1. At this time, 100 μg of BsAb17 was used for booster immunization, and the spleen was taken 2-3 days later. The sequence of BsAb17 is shown in Table 2.
[0105] Table 1 BsAb17 mice immune titer detection data
[0106]
[0107] Tab...
Embodiment 2
[0109] Example 2 Construction of immune library and antibody screening
[0110] 2.1 Phage display immune antibody library construction
[0111] The B lymphocytes in the spleen of the immunized mouse described in Example 1 were isolated, their RNA was extracted, and reverse-transcribed into cDNA by a reverse transcription kit (TaKaRa, 6210A). Design primers to amplify the coding sequences of the light chain variable region and the heavy chain variable region and the first constant region (CL and CH1) respectively, and connect the coding sequence of the M13 phage GIII protein at the 3' end of the coding sequence of CH1, Then cloned into a vector for phage display. Then, the vector was transformed into competent Escherichia coli SS320 cells (Lucigen, MC1061 F) by electroporation (Bio-Rad, MicroPulser). After recovery for 1 hour, the vector was spread on a 2-YT solid plate with ampicillin resistance. By serial dilution plating, the storage capacity of this immune library was det...
Embodiment 3
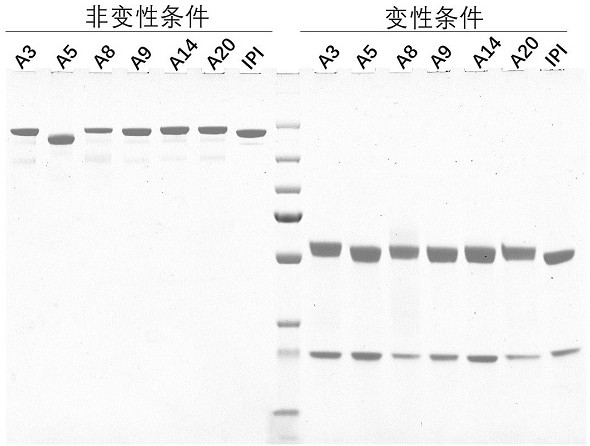
[0127] Example 3 Construction, expression and purification of antibodies
[0128] The 20 murine antibodies obtained in Example 2 were constructed as murine IgG1 subtype, and the amino acid sequences of the light and heavy chain variable regions of the antibodies are shown in Table 4-3 (SEQ ID NO: 1-34).
[0129] 3.1 Plasmid construction
[0130] From the candidate monoclonal strains obtained through screening, PCR amplification was performed to obtain DNA fragments encoding antibody heavy chain and light chain. By means of homologous recombination, the DNA fragments encoding each heavy chain and light chain were respectively constructed on the eukaryotic expression vector plasmid pcDNA3.4 (Invitrogen) containing the mouse IgG1 Fc fragment (SEQ ID NO: 35), and the complete Recombinant plasmids of heavy and light chain full-length genes, wherein the complete heavy chain contains the antibody heavy chain variable region, mouse CH1 fragment (SEQ IDNO: 36) and mouse IgG1 Fc fragme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com