In-vitro culture method for efficiently inducing polarization of macrophages
A technology of in vitro culture of macrophages, applied in the field of biomedicine, can solve the problems of lack of systematic research and insufficient elaboration, and achieve the effect of high stability and high induction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
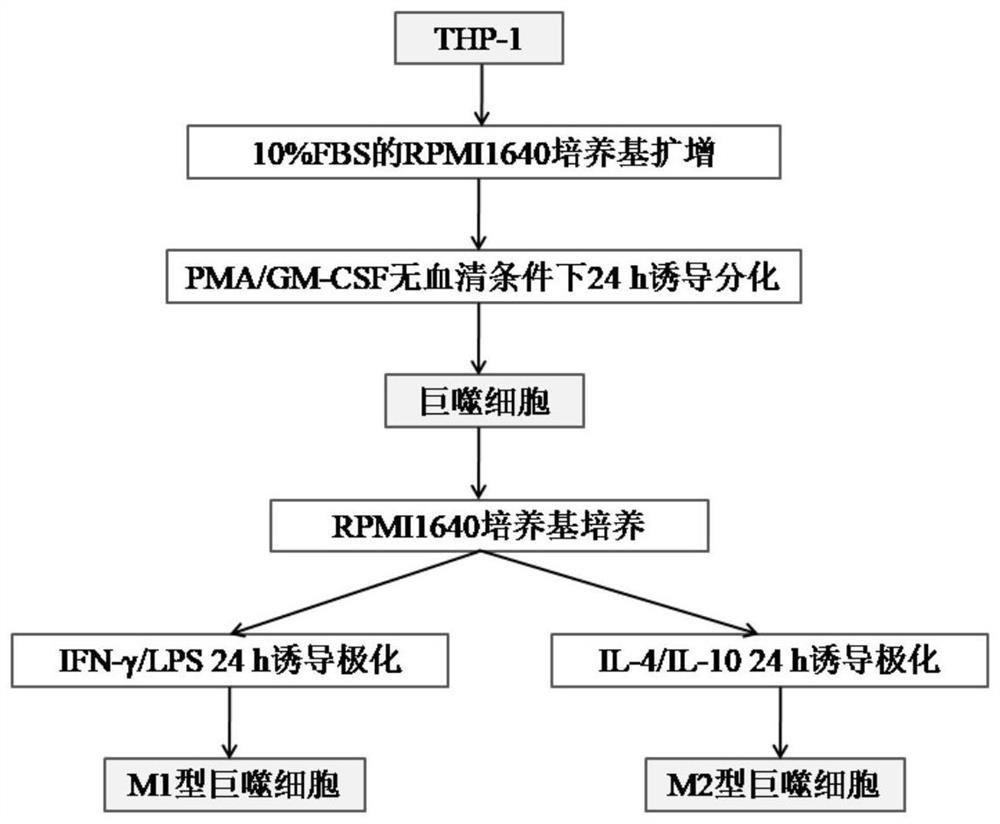
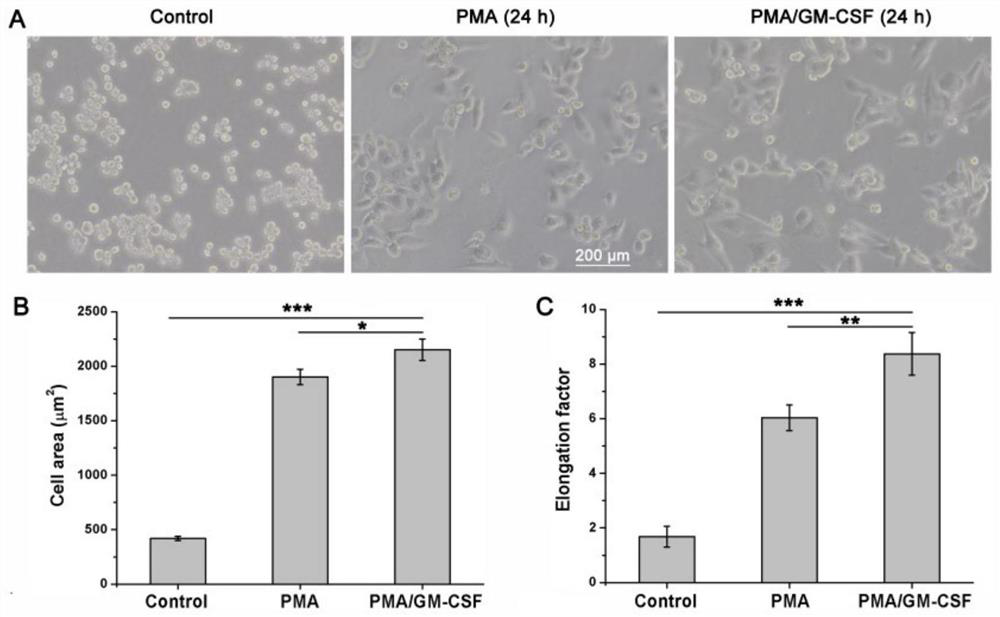
[0032] THP-1 is a monocytic leukemia cell, and monocyte-derived macrophages highly express CD11b, and THP-1 cells undergo significant changes in morphology after being induced to differentiate into macrophages, from nearly round suspension cells to Multi-projectile adherent cells.
[0033] The present invention uses a real-time quantitative gene amplification fluorescence detection system (q-PCR) to detect the expression of CD11b in cells and analyze the morphology of cells to identify the situation in which THP-1 cells are induced to differentiate into macrophages.
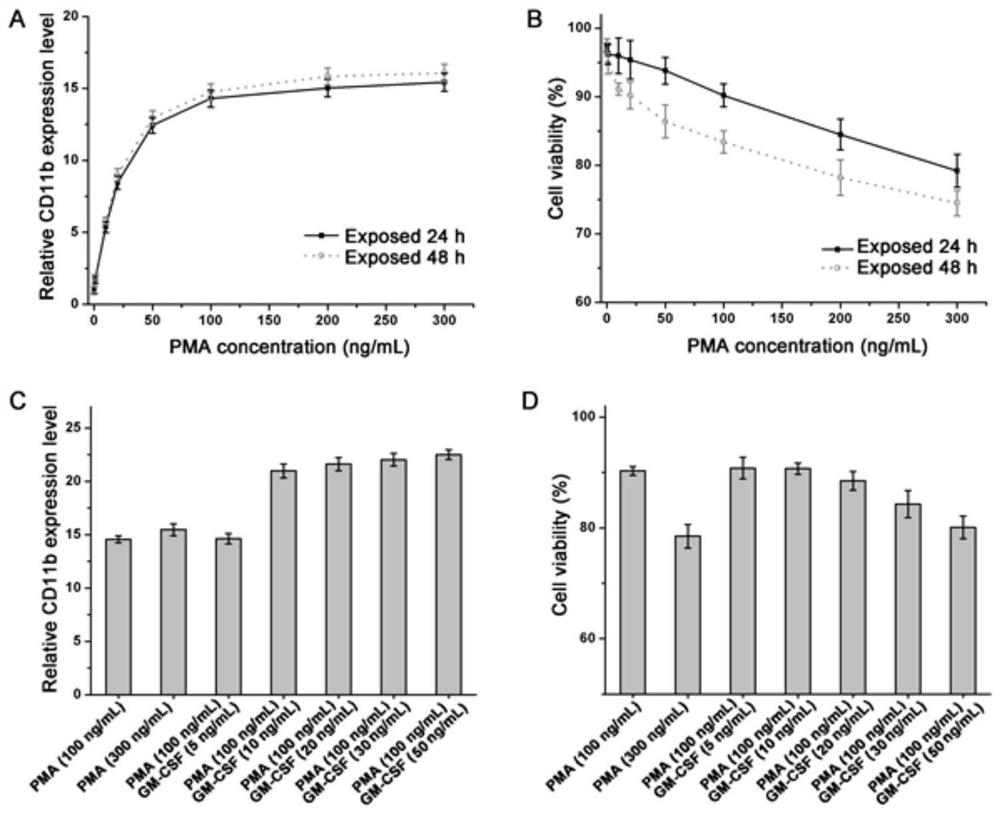
[0034] Firstly, verify the most appropriate dosage of PMA: set up PMA treatment groups with different concentration gradients, blank (PMA concentration 0) as negative control, q-PCR to detect the expression of CD11b in cells, the primer sequences used are shown in Table 1; MTT method is also used for detection cell viability.
[0035] The results of this experiment see figure 2 A and figure 2 B, from figur...
Embodiment 2
[0038] On the basis of the experiment in Example 1, to verify the effect of GM-CSF on THP-1-induced differentiation into macrophages, in the case of adding a certain concentration of PMA and inducing for 24 h, at the same time set the group of adding different concentration gradients of GM-CSF, Detect the expression of CD11b and the survival rate of cells, see the results figure 2 C and figure 2 d. from figure 2 C and figure 2 In D, it can be seen that when the concentration of GM-CSF is higher than 5 ng / mL, the expression of CD11b in cells increases significantly, far exceeding the induction effect of PMA alone. With the further increase of GM-CSF concentration, the cell viability gradually decreases .
[0039] Therefore, it is inferred that 100 ng / mL PMA and 10 ng / mL GM-CSF co-induced the degree of differentiation and cell activity of macrophages obtained by co-induction for 24 h is more suitable, which can be used as the culture conditions for THP-1 cells to induce ...
Embodiment 3
[0044] The present invention designs a method for detecting changes in the level expression of macrophage marker genes by q-PCR to identify macrophage polarization models.
[0045] The present invention screens out 5 genes that are closely related to macrophage polarization: IL-1β, iNOS, Arg1, CD206, and CD86, wherein IL-1β, iNOS, and CD86 are M1-type macrophage surface markers, and Agr1, CD206 is a surface marker of M2 macrophages. The primer sequences designed to amplify the genes are shown in Table 1 below.
[0046] Table 1 Sequences of amplification primer pairs
[0047] Primer name Forward primer (5'->3') Reverse primer (5'->3') CD11b ACTTGCAGTGAGAACACGTATG TCATCCGCCGAAAGTCATGTG IL-1β ATGATGGCTTATTACAGTGGCAA GTCGGAGATTCGTAGCTGGA iNOS TTCAGTATCACAACCTCAGCAAG TGGACCTGCAAGTTAAAATCCC CD206 GCCGGTGACCTCACAAGTAT ACGAAGCCATTTGGTAAACG Agr1 GTGGAAACTTGCATGGACAAC AATCCTGGCACATCGGGAATC CD86 CTGCTCATCTATACACGGTTACC ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com