Preparation method of bis(4-chlorobutyl) ether
A technology for chlorobutyl and dichlorobutane, which is applied in the field of chemical preparation, can solve the problems of troublesome treatment of three wastes, high catalyst price, low product yield, etc., and achieves low cost, control of side reactions, yield and purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0025] A kind of preparation method of two (4-chlorobutyl) ethers, comprises the steps:
[0026] Step 1. Slowly add the chlorinating agent to tetrahydrofuran under low temperature and stirring; control the temperature and add concentrated sulfuric acid dropwise; wherein, the chlorinating agent refers to: thionyl chloride, sulfuryl chloride, phosphorus oxychloride, benzene One of formyl chloride and phosphorus trichloride, especially thionyl chloride or phosphorus oxychloride; the low temperature when adding chlorinating agent is -20~0°C; when adding concentrated sulfuric acid, the temperature is controlled at 0-15°C ; The molar ratio of tetrahydrofuran to chlorinating agent available chlorine is 1:1-1.5; the molar ratio of tetrahydrofuran to concentrated sulfuric acid is 4-6:1.
[0027] Step 2. Add the solvent in the other one in advance. After rising to a certain temperature, start to drop the mixed solution prepared in step 1, and control the temperature during the dropping ...
specific Embodiment 1
[0031] A kind of preparation method of two (4-chlorobutyl) ethers, comprises the steps:
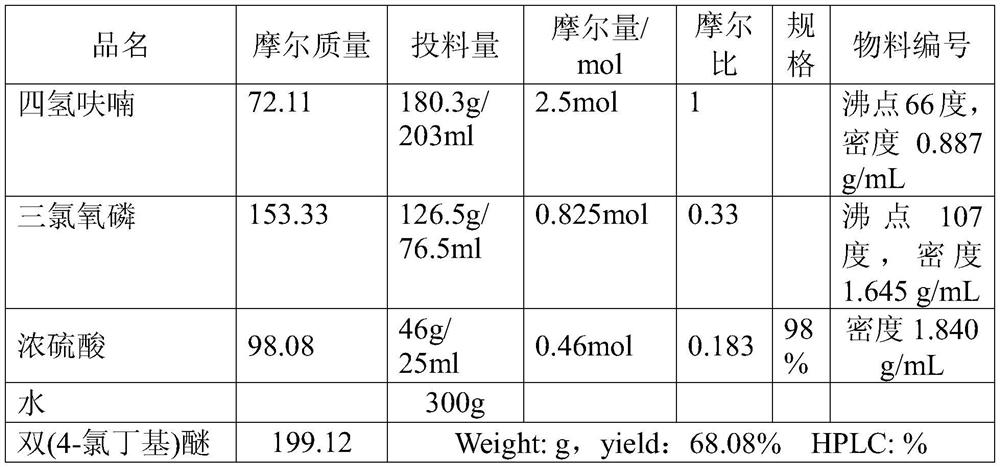
[0032] Step 1. In a 1L three-necked flask, add 180.3g of dried tetrahydrofuran and start stirring. Under ice bath, add 126.5g of phosphorus oxychloride dropwise; cool to 10-15°C, add 46g of concentrated sulfuric acid dropwise, and control the temperature. over 20°C;
[0033] Step 2. Add 400g of 1,4-dichlorobutane into another 2L three-necked bottle, raise the internal temperature to 70°C, add the mixed solution prepared in steps 1 and 2 dropwise, and control the internal temperature at 70-90°C; After adding the mixed solution, lower the temperature to 70°C, add 300g of water, and continue to reflux for 30 minutes;
[0034] Step 3: Azeotropic distillation at atmospheric pressure to remove unreacted tetrahydrofuran and a small amount of by-product 1,4-dichlorobutane formed, with a boiling point of 161-163°C, steaming to a steam temperature of 99-100°C to stop; cooling to room temperature, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com