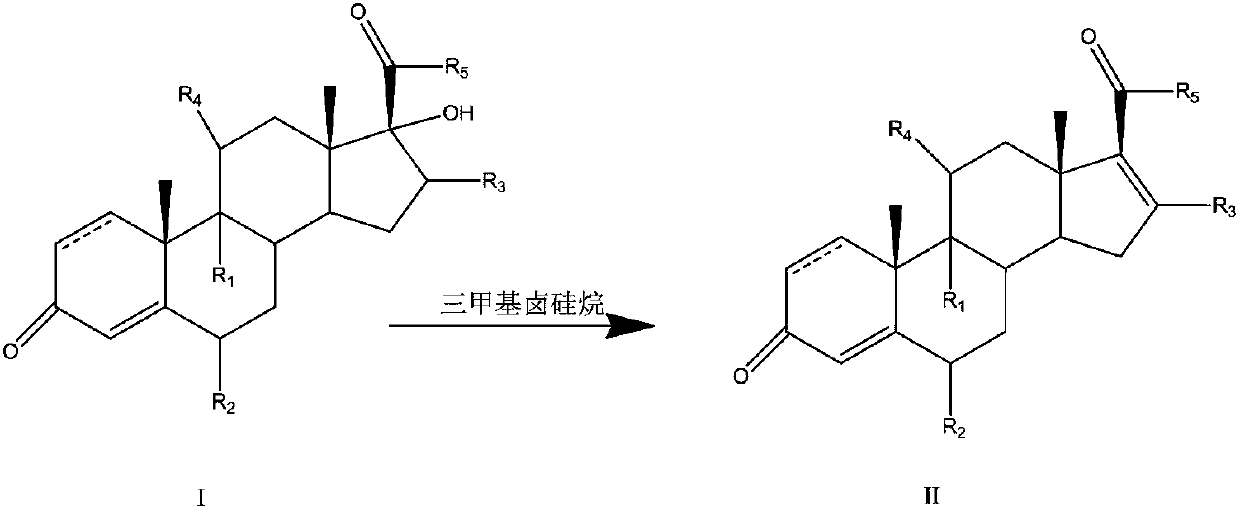
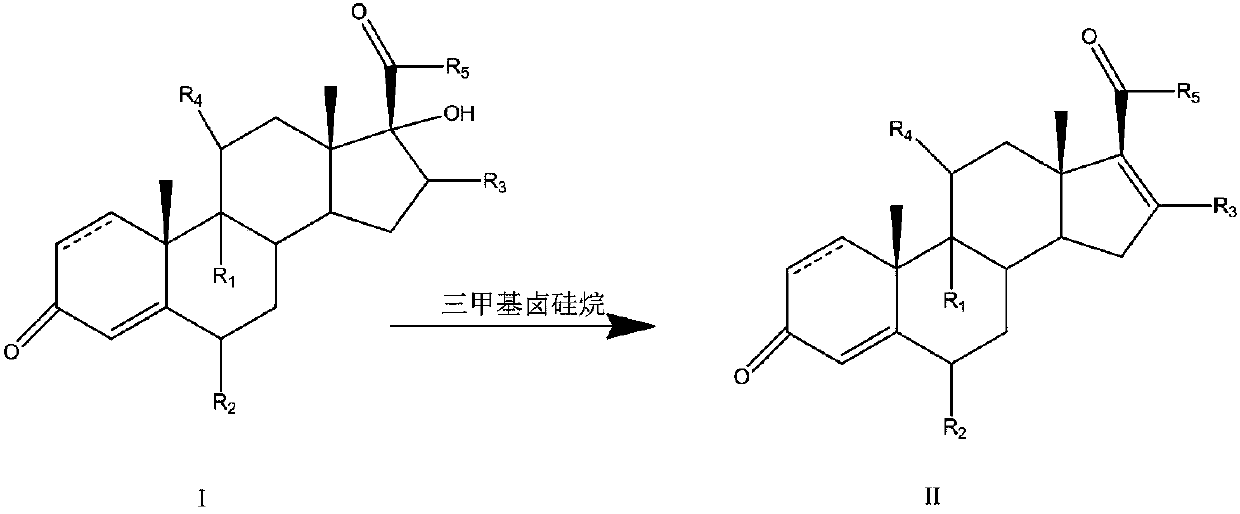
Preparation method of 16-ene steroid compound
A technology for steroids and compounds, applied in the production of steroids, organic chemistry, bulk chemicals, etc., can solve the problems of long reaction time, many reaction steps, and miscellaneous products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
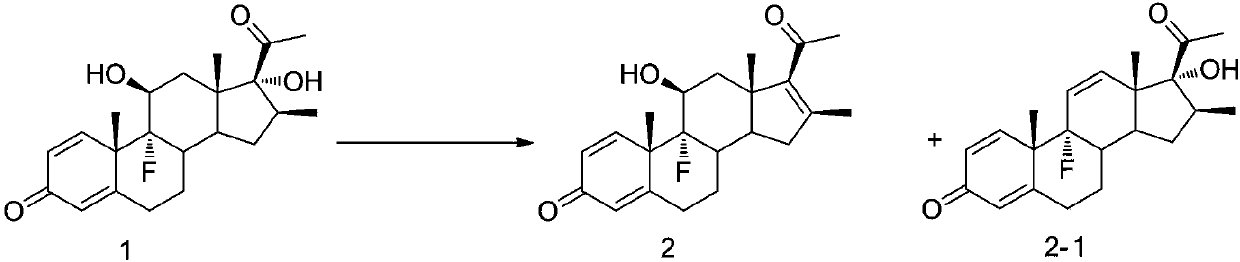
Embodiment 1
[0048]
Embodiment 1-1
[0050]Compound 1 (5g, 13.5mmol) was dissolved in acetonitrile (100ml), trimethyl iodosilane (5.8ml, 40.5mmol) was added, and the reaction was stirred at 50°C. The reaction progress was monitored by TLC until the reaction was complete, and Na 2 S 2 o 3 aq (300ml, 10% w / v) terminated the reaction, separated the liquids, extracted the liquids with 200ml ethyl acetate, combined the organic phases and obtained the white solid 2 (4.4g) by ethyl acetate / petroleum ether recrystallization after vacuum distillation. The purity is 98.5%, and the molar yield is 92%. After testing, a small amount of substance 2-1 is formed in the mother liquor.
Embodiment 1-2
[0052] Compound 1 (5g, 13.5mmol) was dissolved in acetonitrile (100ml), trimethyl iodosilane (3.8ml, 27.0mmol) was added, and the reaction was stirred at 50°C. The reaction progress was monitored by TLC until the reaction was complete, and Na 2 S 2 o 3 aq (300ml, 10% w / v) to terminate the reaction, add 200ml ethyl acetate to extract and separate liquid, and recrystallize through ethyl acetate / petroleum ether after vacuum distillation to obtain white solid 2-1 (4.4g), purity 99.2% , the molar yield is 92%. After detection, a small amount of substance 2 is generated in the mother liquor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com