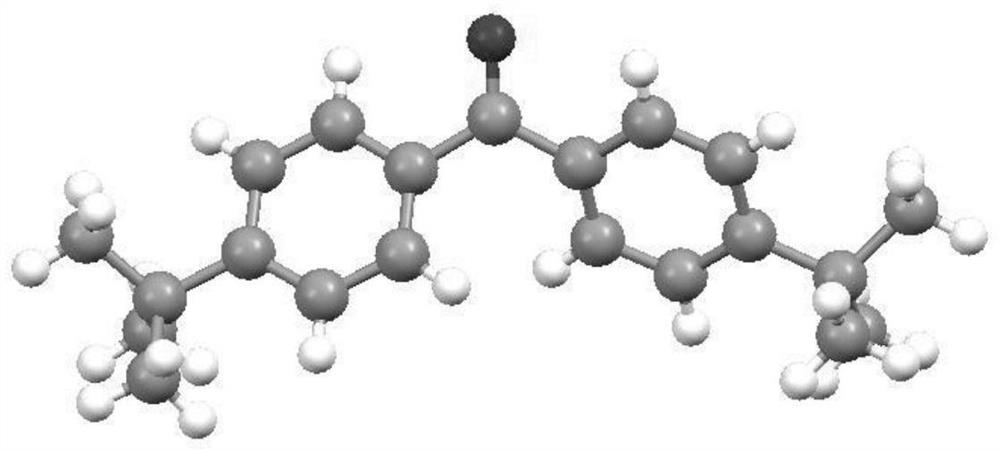
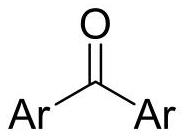
Preparation method of diaryl ketone
A technology of diaryl ketones and aryl groups is applied in the field of preparation of diaryl ketones and achieves the effects of high yield, readily available raw materials and simple and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: aryl is p-tert-butylphenyl, the method for preparing diaryl ketone, the steps are as follows:
[0032] (1) Preparation of 1,1,4,4-tetra-tert-butylphenylbutadiol
[0033] Yield: 65.6%, m.p.: 243-245°C; 1 H NMR (400MHz, Chloroform-d) δ7.37(d, J=8.5Hz, 4H), 7.25(d, J=8.1Hz, 12H), 4.67(s, 2H), 4.47(d, J=4.7Hz ,2H),3.72(d,J=4.5Hz,2H),1.36(s,18H),1.22(s,18H). 13 C NMR (101MHz, Chloroform-d) δ149.9, 149.7, 141.2, 141.1, 141.0, 125.4, 125.2, 124.6, 81.4, 72.4, 34.5, 34.3, 31.4, 31.2.
[0034] (2) Preparation of p-tert-butylphenyl dichlorocyclosulfite
[0035] In a round-bottomed flask, 1,1,4,4-tetra-tert-butylphenylbutanetetraol solution in tetrahydrofuran and 30 equivalents (that is, 1,1,4,4-tetra-tert-butylphenylbutanetetraol The molar ratio of triethylamine to triethylamine is 1:30, the same below), and after stirring at 0°C for 15 minutes, 3 equivalents of thionyl chloride (that is, 1,1,4,4-tetra-tert-tert The molar ratio of butylphenyl butanetetraol to...
Embodiment 2
[0039] Embodiment 2: aryl is p-methylphenyl, the method for preparing diaryl ketone, the steps are as follows:
[0040] (1) Preparation of 1,1,4,4-tetra-p-methylphenylbutanetetraol
[0041] (400MHz,Chloroform-d)δ7.20(s,4H,Ar-H),7.15(s,8H,Ar-H),7.06(s,4H,Ar-H)4.68(s,2H,OH), 4.41(s,2H,CH),3.80(s,2H,OH),2.36(s,6H,CH3),2.24(s,6H,CH3). 13 C NMR (101MHz, DMSO-d 6 )δ143.9, 143.1, 135.8, 135.4, 129.0, 128.5, 126.7, 125.8, 81.4, 71.8.
[0042] (2) Preparation of p-methylphenyl dichlorocyclosulfite
[0043] In a round-bottomed flask, 1,1,4,4- tetrahydrofuran solution of tetra-p-methylphenyl butanetetraol and 30 equivalents of triethylamine were stirred at 0°C for 15 minutes, and then 3 equivalents of chlorinated ethylene chloride were added dropwise. Sulfone, after stirring for 30 minutes, return to room temperature (25-30°C), continue stirring for 30 minutes, add water (remove the triethylamine salt), and recrystallize with ethanol to obtain p-methylphenyl dichlorocyclosulfurous ...
Embodiment 3
[0046] Embodiment 3: aryl is p-trifluoromethylphenyl, the method for preparing diaryl ketone, the steps are as follows:
[0047] (1) Preparation of 1,1,4,4-tetrafluoromethylphenylbutanetetraol
[0048] 32%, m.p.:135-136℃, 1 H NMR(400MHz,Chloroform-d):δ7.66-7.42(m,16H,Ar-H),4.63(s,2H,OH),4.46(s,2H,CH),3.84(s,2H,OH ). 13 C NMR (101MHz, DMSO-d 6 )δ151.0, 149.2, 128.2, 127.0, 125.3, 124.9, 80.8, 70.9.
[0049] (2) Preparation of p-trifluoromethylphenyl dichlorocyclosulfite
[0050] In a round-bottomed flask, 1,1,4,4-tetrafluoromethylphenylbutanol tetrahydrofuran solution and 30 equivalents of triethylamine were stirred at 0°C for 15 minutes, and then 3 equivalents of chlorine were added dropwise. sulfoxide, stirred for 30 minutes and then returned to room temperature (25-30°C), continued to stir for 30 minutes, then added water (to remove the generated triethylamine salt), and recrystallized with ethanol to obtain p-trifluoromethylphenyl dichloride Cyclic sulfites. Ar=4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com