Method for hydrolyzing glycolic acid ester based on carbonic acid system as traceless catalyst
A glycolic acid ester and catalyst technology, applied in the preparation of carboxylate/lactone, reagents, organic chemistry, etc., can solve problems such as environmental hazards, greenhouse effect, CO2 emissions, etc., to avoid strong corrosion and toxicity , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
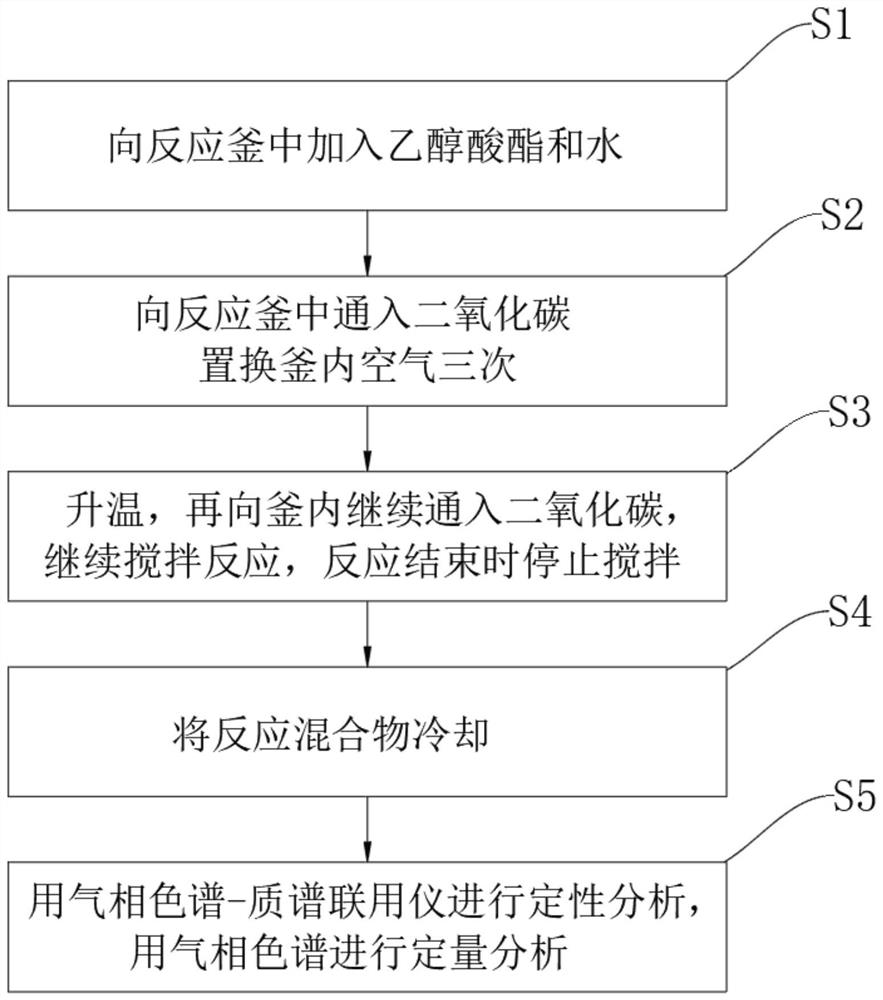
[0025] A 250 mL Hastelloy reactor was charged with 8 g of methyl glycolate (89 mmol) and 9 g of water (500 mmol). The vessel was then tightly sealed and attached to the reactor unit with 2 MPa of CO 2 Purge 3 times, then with CO 2 Saturation until the pressure remains stable at 1.5MPa. While stirring at 800 rpm, the vessel was heated to 100° C., where the reaction was continued for 3 h; the maximum pressure reached at this temperature was 6.5 MPa. After this time, the solution was cooled to ambient temperature, gas was released, and stirring was stopped. The resulting product was then analyzed by GC / MS, which indicated a glycolic acid yield of 65%;
Embodiment 2
[0027] A 250 mL Hastelloy reactor was charged with 8 g of methyl glycolate (89 mmol) and 9 g of water (500 mmol). The vessel was then tightly sealed and attached to the reactor unit with 2 MPa of CO 2 Purge 3 times, then with CO 2 Saturation until the pressure remains stable at 2MPa. While stirring at 800 rpm, the vessel was heated to 120° C., where the reaction was continued for 5 h; the maximum pressure reached at this temperature was 6.5 MPa. After this time, the solution was cooled to ambient temperature, gas was released, and stirring was stopped. The resulting product was then analyzed by GC / MS, which indicated a glycolic acid yield of 85%.
Embodiment 3
[0029] A 250 mL Hastelloy reactor was charged with 8 g of methyl glycolate (89 mmol) and 9 g of water (500 mmol). The vessel was then tightly sealed and attached to the reactor unit, purged 3 times with 2 MPa of CO , then with CO 2 Saturation until the pressure remains stable at 2MPa (water absorbs a considerable amount of CO 2 ). While stirring at 800 rpm, the vessel was heated to 150° C., where the reaction was continued for 3 h; the maximum pressure reached at this temperature was 7.5 MPa. After this time, the solution was cooled to ambient temperature, gas was released, and stirring was stopped. The resulting product was then analyzed by GC / MS, which indicated a 90% yield of glycolic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com