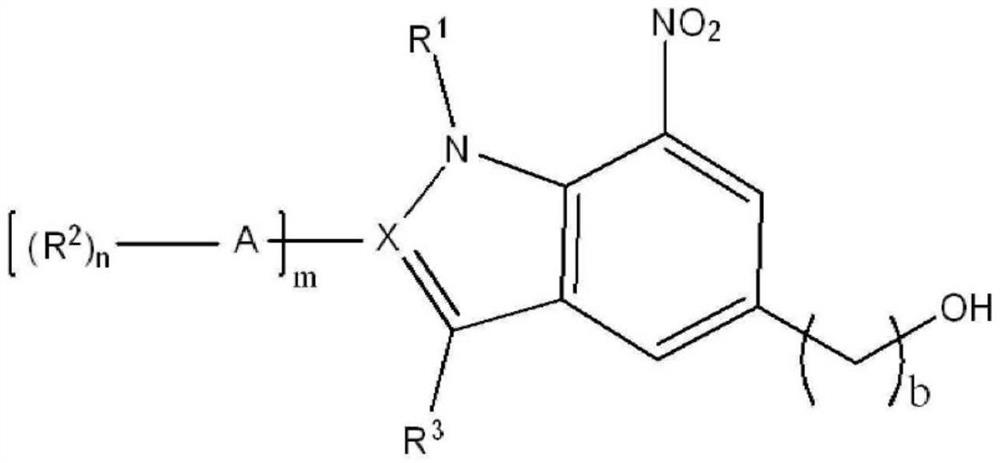
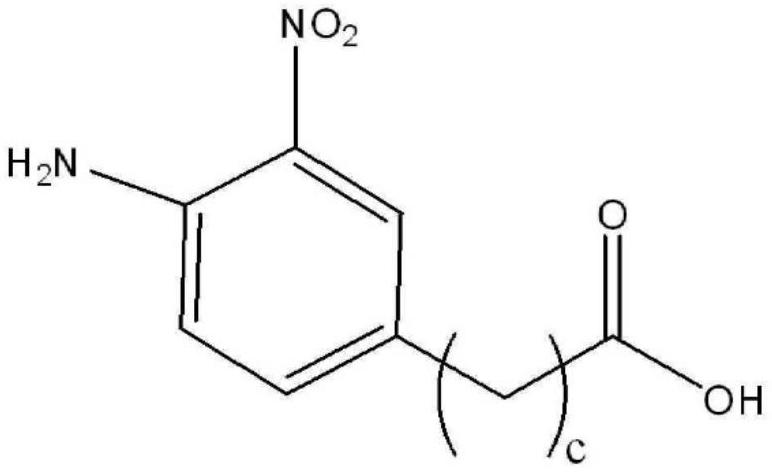
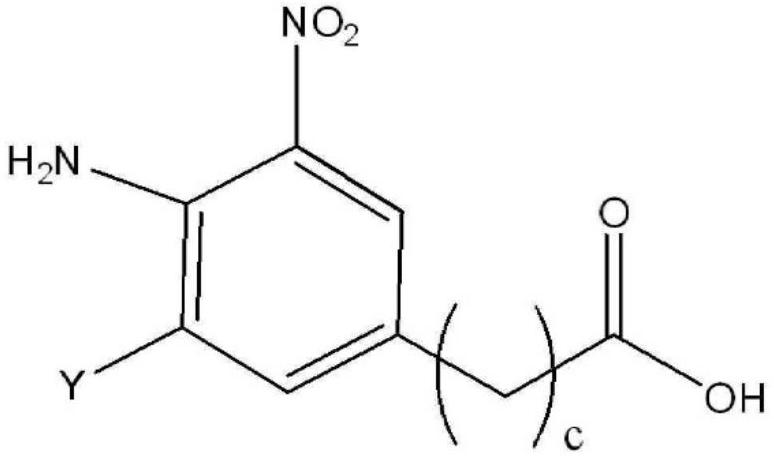
Method for producing indole or indazole compound
A compound and halogen technology, applied in the field of preparing nitro-containing indole or indazole compounds, can solve the problems of difficult scale-up of column purification process, complicated process steps, poor process efficiency, etc., to reduce the ratio of by-products, simplify the Process, the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Embodiment 1: the synthesis of (7-nitro-2-phenyl-1H-indol-5-yl)methanol
[0129] 1) Synthesis of 4-amino-3-iodo-5-nitrobenzoic acid
[0130] Add commercially available 4-amino-3-nitrobenzoic acid (manufactured by Sinochem Ningbo Co., Ltd. (China)) (5.0 kg), NIS (N-iodosuccinimide, 9.3 kg) to the reactor ), H 2 SO 4 (0.50 kg) and THF (25.0 L) and stirred at room temperature, then the reactor was heated to a temperature of 80° C. and stirred for 2 hours. In this case, the reaction was terminated when the analysis result of the reaction mixture by HPLC reached 5.0% or less of the peak of 4-amino-3-nitrobenzoic acid.
[0131] After confirming that the reaction was complete, the temperature of the reactor was cooled to room temperature, and then dichloromethane (DCM, 50.0 L) was added to the concentrated solution obtained by distillation under reduced pressure to form a solid. The solid formed was stirred at room temperature for 1 h or more, filtered and washed [1st: DCM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com