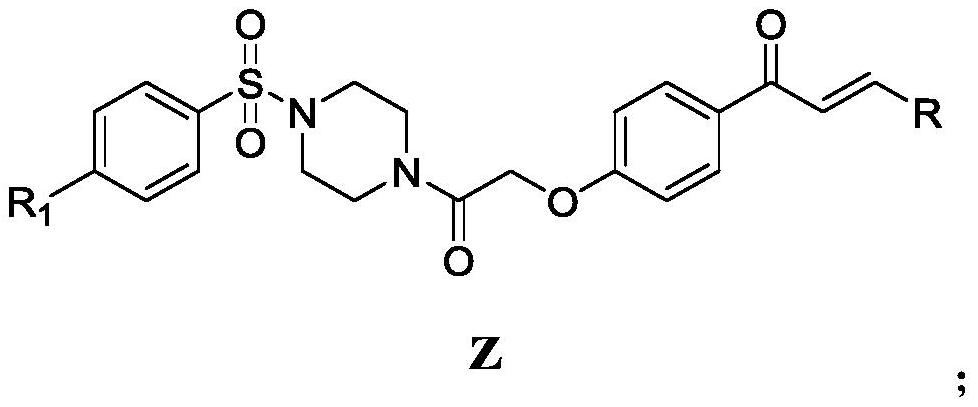
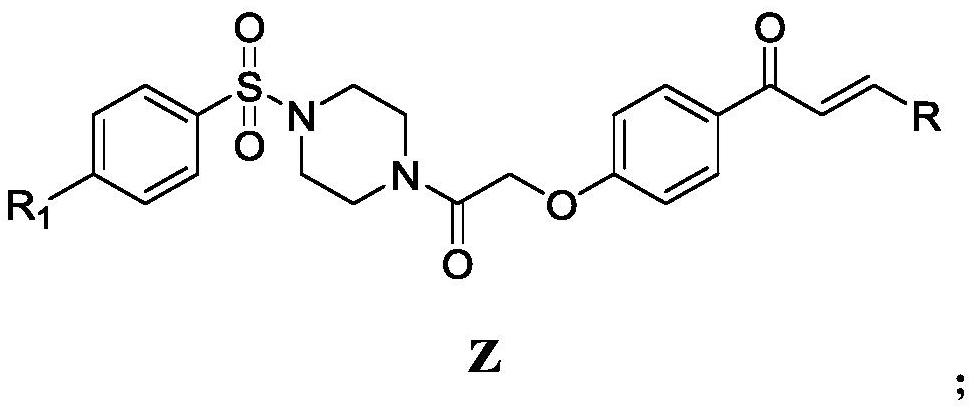
Chalcone derivative containing sulfonyl piperazine as well as preparation method and application thereof
A technology for chalcone derivatives and sulfonylpiperazine, which is applied in the field of chalcone derivatives containing sulfonylpiperazine and its preparation, can solve problems such as unreported, and achieve the effect of inhibiting the activity of plant bacteria and fungi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
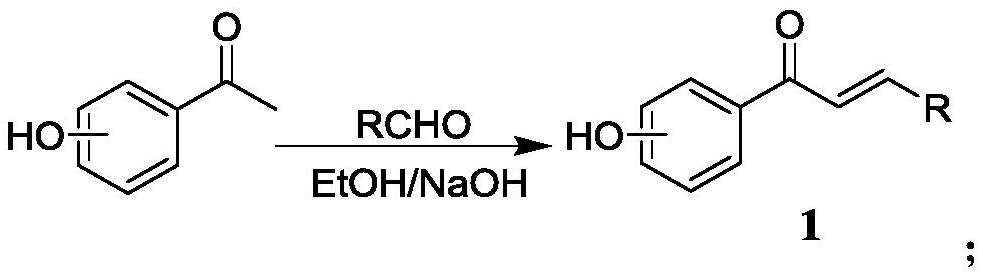
[0039] Step 1, the preparation of 3-(2-chlorophenyl)-1-(4-hydroxyphenyl)-2-propen-1-one (intermediate 1):
[0040] Add 1.5g p-hydroxyacetophenone (11.02mmol) and 1.86g 2-chlorobenzaldehyde (13.22mmol) in a 100mL three-necked flask, then add 25mL (30.05mmol) ethanol to fully dissolve the reactant, then add 35mL 5% of NaOH (30.05 mmol) solution. Magnetic stirring, under ice bath conditions for 12 hours until the reactant reacts completely, monitor with TLC until the raw material point disappears, pour the reaction system in the three-necked flask into a 500mL ice bath beaker, add 5% HCl drop by drop with a rubber dropper solution, until the pH value in the beaker was measured to be about 6 with pH test paper, a large amount of white solids were precipitated at the bottom of the beaker as intermediate 1, filtered by suction, and dried in a far-infrared drying oven (TIR-X31 type ) 8h (8-48h can be), standby, yield: 92%.
[0041] Step 2, the preparation of 1-((4-chlorophenyl)sulf...
Embodiment 2
[0048] Step 1, the preparation of 1-(4-hydroxyphenyl)-3-(pyridin-2-yl)-2-propen-1-one (intermediate 1):
[0049] Same as step 1 of Example 1, the only difference is that 2-chlorobenzaldehyde is replaced by 2-pyridinecarbaldehyde in an equimolar amount.
[0050] Step 2, the preparation of 1-((4-chlorophenyl)sulfonyl)piperazine (intermediate 2):
[0051] Same as Step 2 of Example 1.
[0052] Step 3, preparation of 2-chloro-1-(4-((4-chlorophenyl)sulfonyl)piperazin-1-yl)ethan-1-one (intermediate 3):
[0053] Same as Step 3 of Example 1.
[0054] Step 4, 1-(4-(2-(4-((4-chlorophenyl)sulfonyl)piperazin-1-yl)-2-oxoethoxy)phenyl)-3-(pyridine- Preparation of 2-yl) prop-2-en-1-one (target compound Z2):
[0055] Same as step 4 of Example 1, the only difference is that 3-(2-chlorophenyl)-1-(4-hydroxyphenyl)-2-propen-1-one is replaced by an equimolar amount of 1-(4 -Hydroxyphenyl)-3-(pyridin-2-yl)-2-propen-1-one. Yield: 69%.
Embodiment 3
[0057] Step 1, the preparation of 1-(4-hydroxyphenyl)-3-(pyridin-4-yl)-2-propen-1-one (intermediate 1):
[0058] Same as step 1 of Example 1, the only difference is that 2-chlorobenzaldehyde is replaced by 4-pyridinecarbaldehyde in an equimolar amount.
[0059] Step 2, the preparation of 1-((4-chlorophenyl)sulfonyl)piperazine (intermediate 2):
[0060] Same as Step 2 of Example 1.
[0061] Step 3, preparation of 2-chloro-1-(4-((4-chlorophenyl)sulfonyl)piperazin-1-yl)ethan-1-one (intermediate 3):
[0062] Same as Step 3 of Example 1.
[0063] Step 4, 1-(4-(2-(4-((4-chlorophenyl)sulfonyl)piperazin-1-yl)-2-oxoethoxy)phenyl)-3-(pyridine- Preparation of 4-yl) prop-2-en-1-one (target compound Z3):
[0064] Same as step 4 of Example 1, the only difference is that 3-(2-chlorophenyl)-1-(4-hydroxyphenyl)-2-propen-1-one is replaced by an equimolar amount of 1-(4 -Hydroxyphenyl)-3-(pyridin-4-yl)-2-propen-1-one. Yield: 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com