Fluorene spirotriphenylamine derivative compound as well as preparation method and application thereof
A technology of fluorene spiro triphenylamine and derivatives is applied to the fluorene spiro triphenylamine derivative compound. In the field of preparation of the compound, problems such as low efficiency can be solved, and the effect of good application prospect, fast RISC rate and enhanced RISC rate can be achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
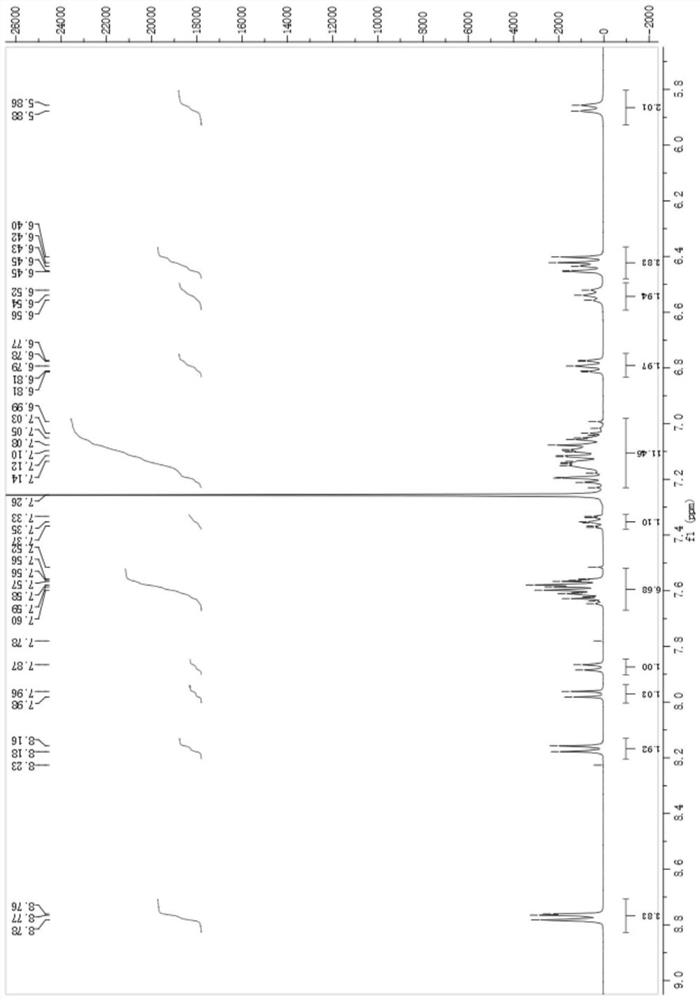
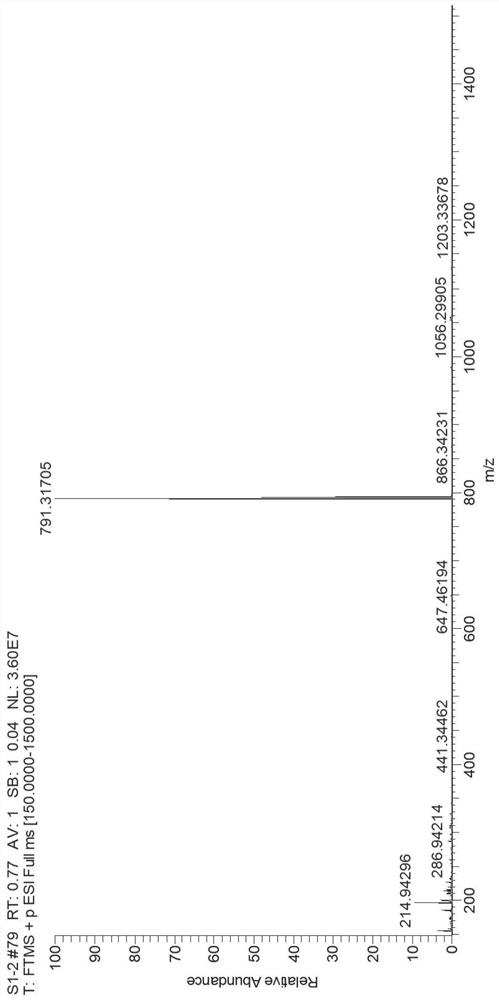
[0056] The present embodiment provides a fluorene spirotriphenylamine derivative compound, the structural formula is shown in M1:
[0057]
[0058] The preparation method of this compound is as follows:
[0059]
[0060] S1. Preparation of 2-chloro-4-iodo-[1,1'-biphenyl]-3-carboxylic acid:
[0061] In a 250 mL flask, combine 2-bromo-3-methylbenzoic acid (4.59 g, 21.3 mmol), palladium acetate (0.24 g, 1.06 mmol), iodobenzenediacetic acid (6.68 g, 21.3 mmol) and iodine (5.42 g) , 21.3 mmol) was dissolved in N,N-dimethylformamide (50 mL). After stirring at 100 °C for 24 h, the reaction mixture was cooled to room temperature and diluted with 50 ml of ethyl acetate. 200 ml of aqueous hydrochloric acid (0.5N) was added to wash off N,N-dimethylformamide, and the crude product was extracted with ethyl acetate. 3 times. The organic phase was collected, washed with saturated brine, and dried over anhydrous sodium sulfate. Concentrate in vacuo at 0~700mbar by rotary evaporator fo...
Embodiment 2
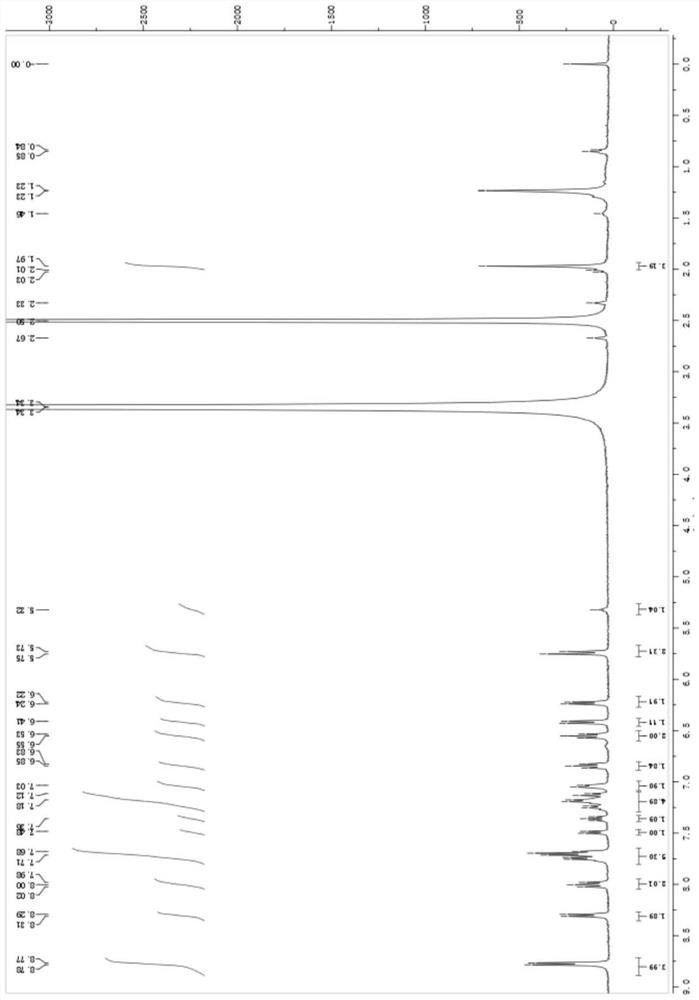
[0073] This embodiment provides a fluorene spirotriphenylamine derivative compound, the preparation method of which is the same as that of embodiment 1, the difference lies in that, different from embodiment 1, the benzoic acid derivative used in S1 is The fluorene spirotriphenylamine derivative compound M2 is obtained, and the structural formula of M2 is shown below.
[0074]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com