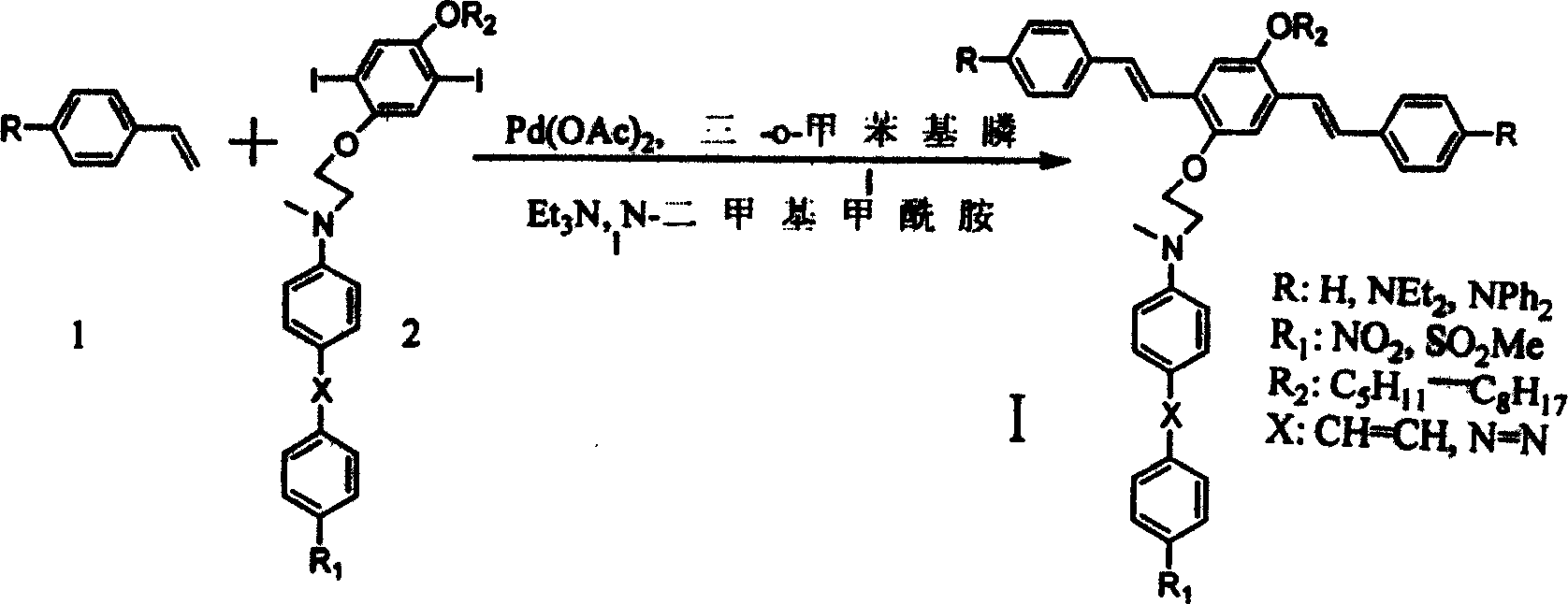
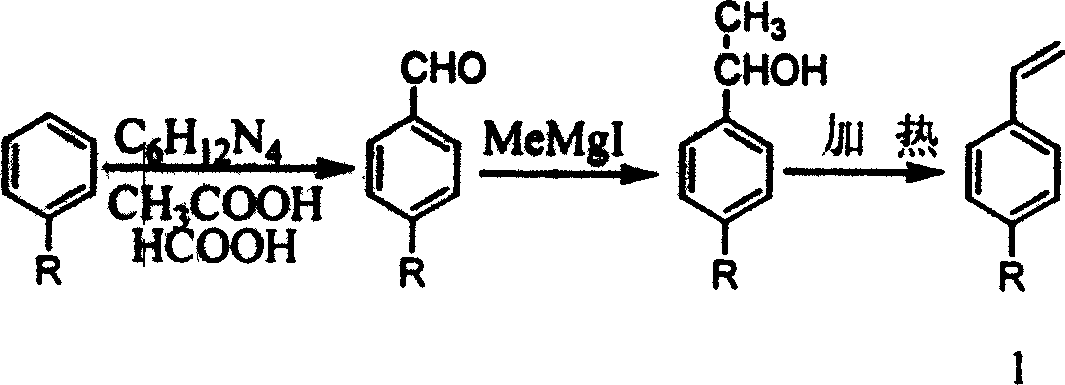
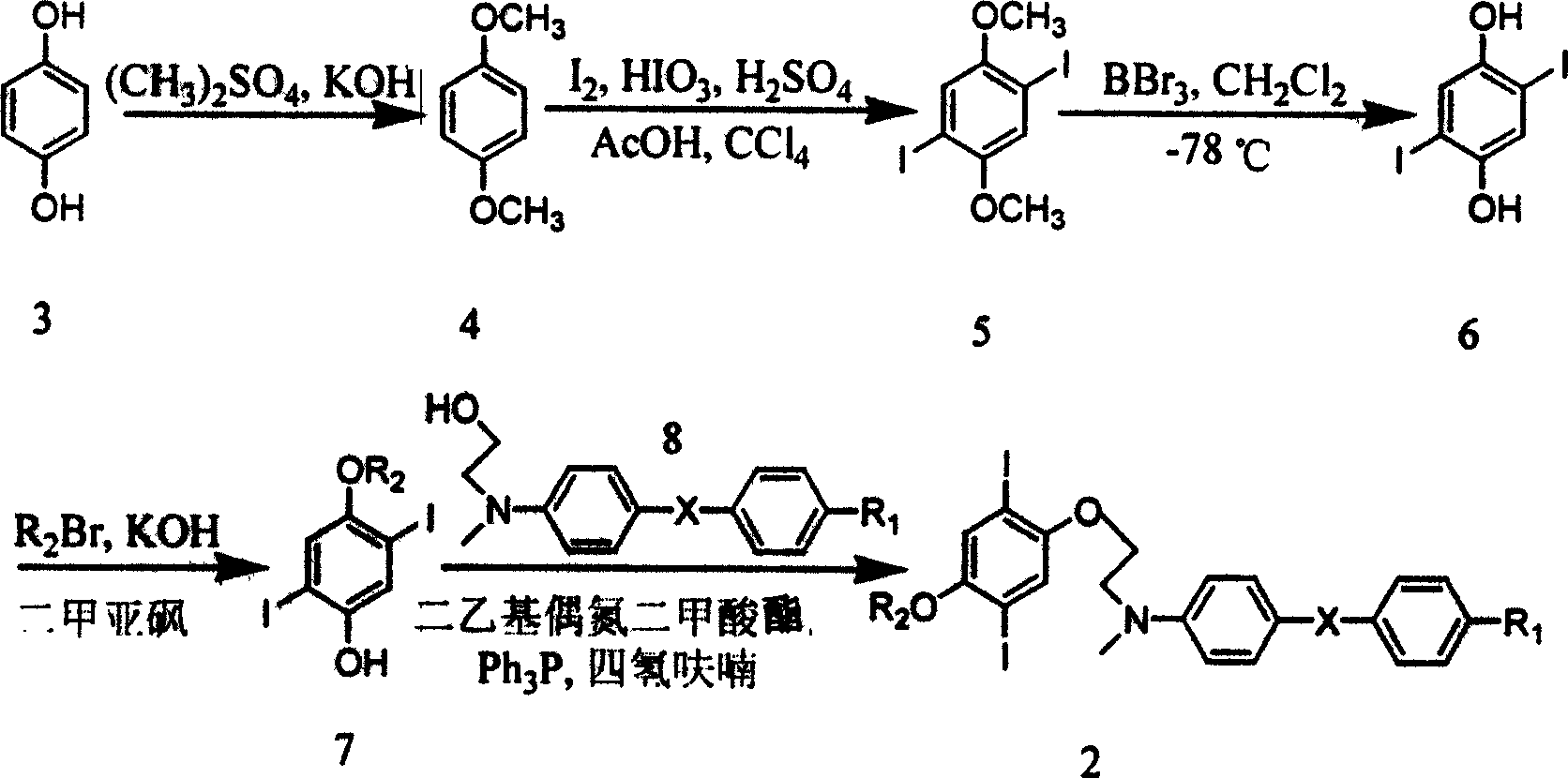
Process for synthesizing broad-band double-photon absorbing molecule in dual-conjugation system
A technology of two-photon absorption and conjugated systems, applied in organic chemistry methods, organic chemistry, etc., to achieve the effects of large two-photon absorption coefficient, wide absorption wavelength range, and ease of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] Step one 1, the preparation of 4-dimethoxybenzene 4
[0020] Under vigorous stirring, dimethyl sulfate (126.2 g, 1.0 mol) was added dropwise to a suspension of hydrobenzenediol 3 (55 g, 0.5 mol) and 10% potassium hydroxide solution (700 mL), and the rate of addition was controlled so that The temperature of the reaction mixture did not exceed 40°C and was then heated to reflux for 30 minutes. The oil layer was separated by cooling, washed with 2M NaOH solution (200ml×2) and water (200ml×2). Methanol was recrystallized to give 1,4 dimethoxybenzene 4 (40 g, mp 56°C).
[0021] Step 2 Preparation of 1,4-dimethoxy-2,5-diiodobenzene 5
[0022] With 1,4-dimethoxybenzene (6.90g, 50mmol), I 2 (5.72g, 45mmol), HIO 3 (5.25g, 30mmol), 30% H 2 SO 4 (15mL), with CCl 4 (20mL) was dissolved in 90mL of acetic acid, the mixture was heated to 75°C and reacted for 3h. The reaction solution was cooled with ice, and crystals were precipitated, filtered and washed with a large amount ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com