Process method for synthesizing Alzvudine
A process method and intermediate technology, applied in the synthesis of pyrimidine nucleosides and in the field of organic chemistry, can solve the problems of cumbersome reaction steps, cumbersome product refining, troublesome post-processing, etc., and achieve simple operation, environmental protection, and reduction of reaction steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
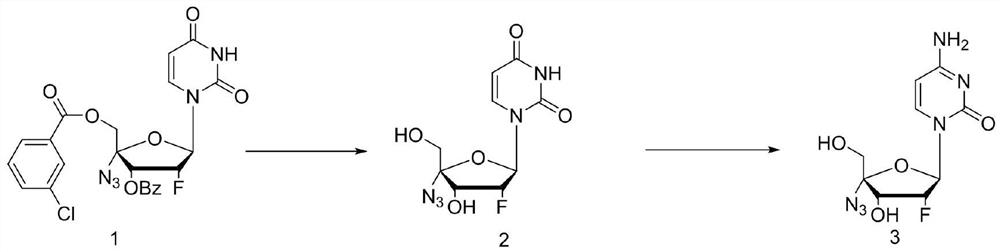
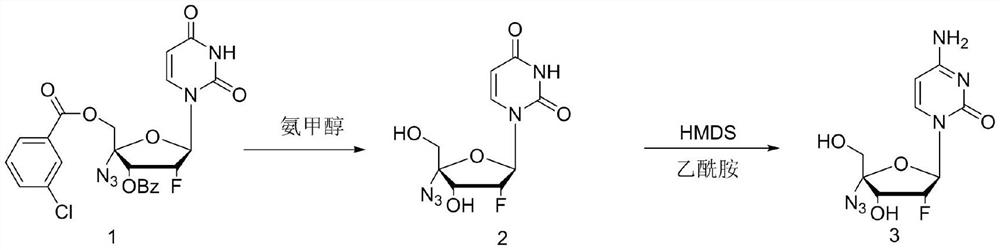
[0029] In the first step, add 2'-fluoro-4'-azido-uridine (20g, 0.038mol) into the reactor, then add 25% ammonia / methanol 100mL, heat up to 50-60°C for 5h, The liquid phase was tracked until the raw materials basically disappeared, the reaction solution was concentrated to a solid, and 60 mL of 75% ethanol was added to crystallize to obtain 9.8 g of the intermediate, with a yield of 89.5%.
[0030] In the second step, add intermediate 2 (9.8g, 0.034mol), hexamethyldisilazane (15g, 0.093mol) and acetamide (5.49g, 0.093mol) into the reactor, and heat up to 120°C for reaction After 24 hours, the reaction was tracked by liquid phase HPLC until the raw material 2 dropped below 1%. The reaction solution was lowered to room temperature, and 50 mL of methanol was slowly added, a large amount of white solids were precipitated, and 6.9 g of the crude product was obtained by suction filtration and drying, and 5.72 g (0.02 mol) of the finished product Azvudine was obtained by r...
Embodiment 2
[0032]
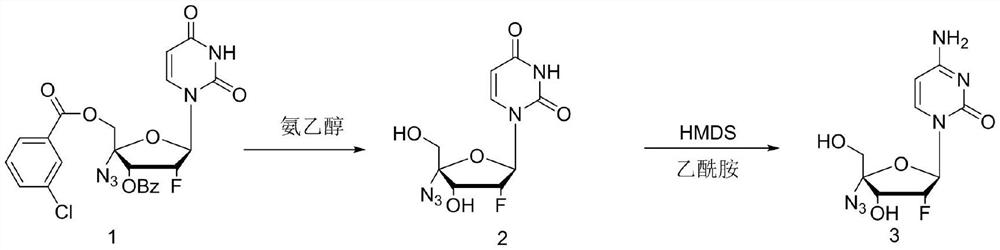
[0033] In the first step, add 2'-fluoro-4'-azido-uridine (20g, 0.038mol) into the reaction kettle, then add 25% ammonia / ethanol 100mL, heat up to 50-60°C for 5h, The liquid phase was tracked until the raw materials basically disappeared, the reaction solution was concentrated to a solid, and then 60 mL of 75% ethanol was added to crystallize to obtain 9.1 g of an intermediate with a yield of 84.2%.
[0034] In the second step, in the reactor, add intermediate 2 (9.1g, 0.032mol), hexamethyldisilazane (14.2g, 0.088mol) and acetamide (5.19g, 0.088mol), and raise the temperature to 120°C After 24 hours of reaction, liquid phase HPLC followed the reaction until the raw material 2 dropped below 1%. The reaction solution was lowered to room temperature, and 50 mL of methanol was slowly added, a large amount of white solids were precipitated, and 6.2 g of the crude product was obtained by suction filtration and drying, and 5.2 g (0.018 mol) of the finished product Azvudine...
Embodiment 3
[0036]
[0037] In the first step, add 2'-fluoro-4'-azido-uridine (20g, 0.038mol) and 100mL of methanol into the reactor, then add sodium methoxide (4.86g, 0.09mol), and heat up to 50 React at -60°C for 5 hours, track the liquid phase until the raw materials basically disappear, concentrate the reaction solution to a solid, and then add 60 mL of 75% ethanol to crystallize to obtain 8.2 g of the intermediate, with a yield of 75.2%.
[0038] In the second step, add intermediate 2 (8.2g, 0.028mol), hexamethyldisilazane (12.4g, 0.077mol) and acetamide (4.55g, 0.077mol) in the reaction kettle, and raise the temperature to 120°C After 24 hours of reaction, liquid phase HPLC followed the reaction until the raw material 2 dropped below 1%. The reaction solution was lowered to room temperature, and 50 mL of methanol was slowly added, a large amount of white solids were precipitated, and 5.8 g of the crude product was obtained by suction filtration and drying, and 4.8 g (0.016 mol) o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com