Method for full-liquid-phase synthesis of leuprorelin
A liquid-phase synthesis technology of leuprolide, applied in the field of medicine, can solve the problems of low purity of crude product, use of many solvents, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] 1. Synthesis of compound 1: Fmoc-Trp(Boc)-Ser(tBu)-Tyr(tBu)-OH
[0071] 1.1 Feeding:
[0072] Feed according to the materials in Table 2.
[0073] Table 2 Types and dosage of materials
[0074]
[0075]
[0076] 1.2 Operation process
[0077] After the synthesized Fmoc-Trp(Boc)-Ser(tBu)-OSu was completely dissolved in DMF, the H-Tyr(tBu)-OH was accurately weighed and added to the above reaction flask, and TEA 110mmol was added to start the reaction. After stirring and reacting for 60 min, HPLC detected that the reaction was complete.
[0078] The reaction solution was poured into the Erlenmeyer flask twice, and then 0.5M hydrochloric acid was added to rapidly stir and precipitate, and the filtered solid was then washed with purified water until neutral, and dried at 30°C. Collect the solids into containers and weigh.
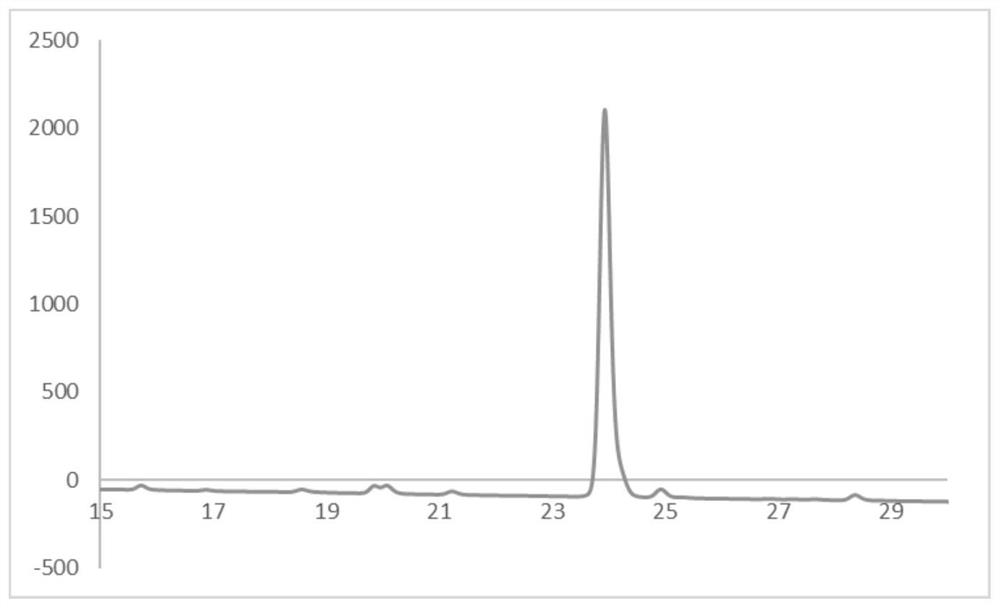
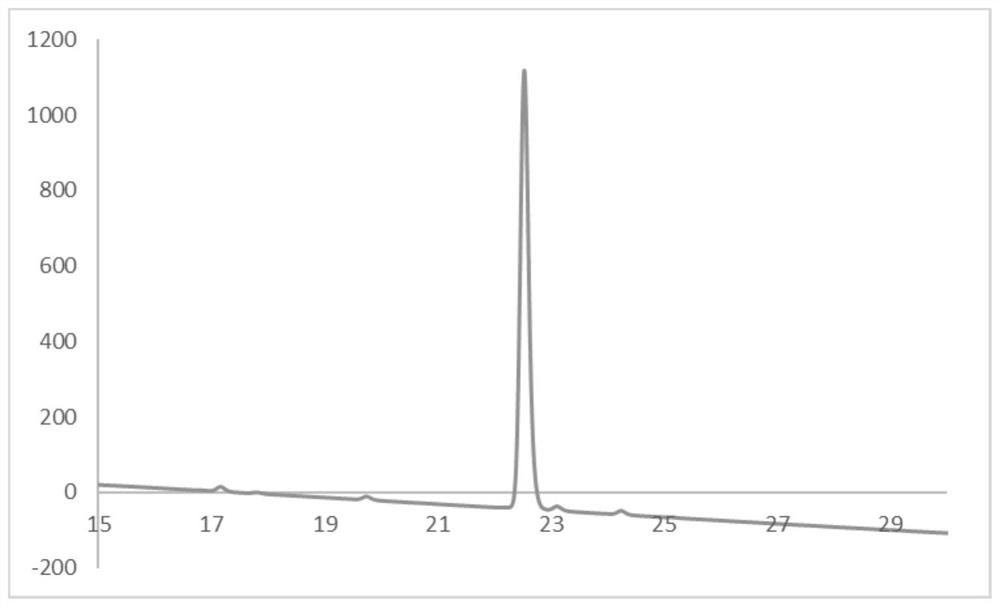
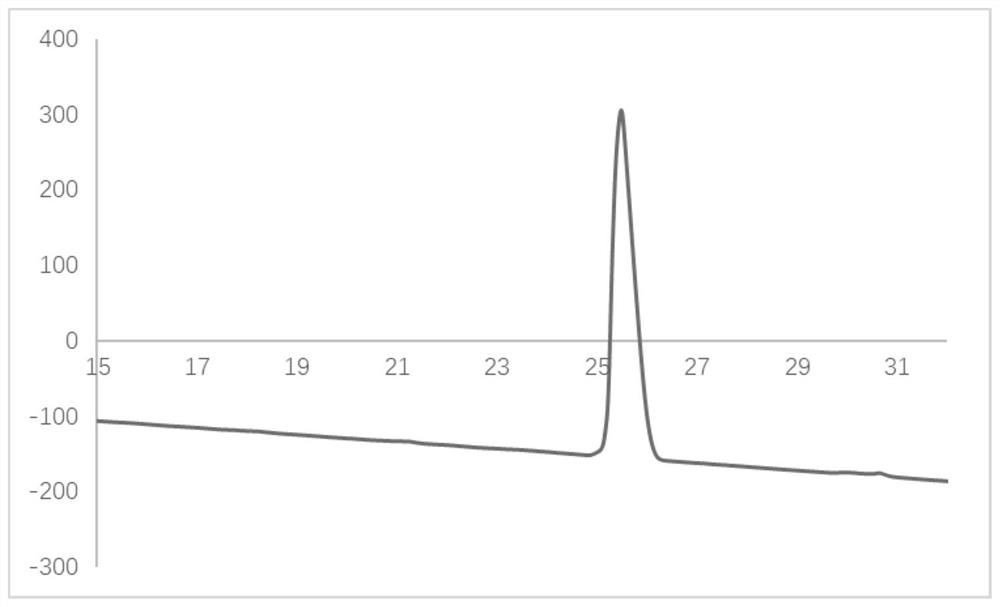
[0079] The obtained compound 1 was analyzed by HPLC, and the liquid chromatogram was obtained as figure 1 shown.
[0080] The analysis metho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com